Introduction
Atrial fibrillation, the commonest arrhythmia in clinical practice, has a fatal ending leading to a rise in mortality. Sudden death, congestive heart failure and stroke are the usual consequences of atrial fibrillation and all are fatal or associated with a poor quality of life (1). The situation and understanding of the illness has not changed much from when James Mackenzie described the illness one hundred years ago when it had shown an absent ‘a’ wave in the jugular pulse and atrial paralysis.
The costs involved in the management and prevention of AF have been described in extreme terms (1). The huge financial burden on the nation needs to be levered away through sincere efforts at finding a solution for the prevention and management of AF. The attempts of past researchers over the last 20 years have not made any significant impact in this direction. The many reasons which have been suggested could have contributed to the pitiable condition that AF management is in today. One may be that clinical presentations of the illness varied from patient to patient and confused the attending physician or cardiologist.
The mechanism of evolution of the disease and the pathophysiology are yet a mystery and could be another reason for the inadequate research and therapy (2). Moreover the classification of AF kept changing; the dearth of a uniform classification could have jeopardized the search for an effective therapy. Previously AF was a single entity and now it is a heterogenous group of illnesses. In 2001 practice guidelines had been chalked out by the Cardiology experts of International standing hoping to improve the patient care, influencing their outcomes and containing the costs of treatment (3).
The American and European organisations dealing with the heart have jointly brought out these guidelines. The National Health Services in the UK now have the NCC-CC Guidelines published in 2006 by the Royal College of Physicians in 2006 to help diagnose and manage AF. They have a similar classification of the varieties of AF and their specific management protocols in differing situations. The results of the previous therapies and researches could not be compared on the same footing due to the varying classifications; the future research may bode well for the management of AF.
The uniformity of classification and therapy could help in comparing results wherever the research is done. A reliable cure is still being investigated. A better understanding of the mechanism would result in a more specific management for that mechanism. This writer will search for relevant research articles to collect ideas on how to approach this illness of AF, its management problems and the solutions as of today.
Significance of the issue
A high level of morbidity and mortality is associated with AF. Considering the costs inevitable to manage this large population, looking for preventive aspects seems a better prospect. The society will benefit tremendously alongside of the economic advantage. Atrial implantation devices and ablation techniques have not produced a uniform beneficial effect in all patients. Therapy with anti-fibrillatory drugs seems to be fairly good. The high risk for mortality and morbidity in atrial fibrillation has been projected as a major global health issue (4). Stroke and cardiomyopathy caused by tachycardia are the two common causes of illness and death.
The common risk factors for stroke are “Atrial fibrillation, hypertension, congestive heart failure and coronary artery disease” (4) AF increases the risk of stroke by 2 to 7 times (4). Strokes following atrial fibrillation are more fatal and account for 12 % of fatal cases a year (4). Concerted efforts need to be made to find a protocol for preventive management of atrial fibrillation and successful cardioversion for patients with AF, reducing the morbidity and mortality of AF, preventing the complications and improving the quality of life of patients with AF.
Definition
The NCC_CC Guidelines of 2006 which has been prepared for utilization in the National Health Services of the United Kingdom has defined “atrial fibrillation as an atrial tachyarrhythmia characterized by predominantly uncoordinated atrial activation with consequent deterioration of atrial mechanical function” (3). The ACC/AHA guidelines of 2001 which were drawn up at the instance of the American College of Cardiology, the American Heart Association and the European Society of Cardiology Committee had defined AF slightly differently “as a supraventricular tachycardia characterized by uncoordinated atrial activation with consequent deterioration of atrial mechanical function” (5).
ECG findings
The electrical activity which produced fragmented and repetitive signals arising from many reentrant waves occurring simultaneously in the atria causes the fibrillary waves which appeared as the ECG finding (6). These waves were seen in rapid oscillatory succession, showing variations in time, shape and size with intermittent, infrequent ventricular responses; the normal p waves were absent (3). The waves could be fine or coarse. The irregular p waves occurred due to irregular electrical signals from the sinu-atrial node. The ventricular response to the AF was seen if the AV node was intact and was 140-150 beats per minute; in addition the vagal and sympathetic tone needed to be fine and drugs should be acting (6). Inhibition of the pacemaker was necessary to have the AF activity revealed in a patient who used a pacemaker (3).
If there was basically a bundle branch block, a wide QRS complex was noticed. If the beats were more than 200 per minute, an accessory pathway could be present. RR (regular relative risk) intervals could occur if there was a heart block either due to conduction disease or a drug (3).
Symptoms
The patients presented with any of the different symptoms: “palpitations, chest pain, dizziness or loss of consciousness” (3). Occasionally the patient was asymptomatic.
Electrophysiological basis of atrial fibrillation
This writer will begin with a simple explanation of the functioning of the heart and the electrophysiological basis of the activity and then progressing towards the more complex ones. The electrical signals began in the sinu-atrial node in the right atrium at the top of the heart and spreads downwards (7). This caused contraction of the heart muscle and simultaneous pumping of the heart. The number of signals per minute was equal the heart rate per minute. The path of the signal was through both atria causing their contraction and pumping of blood from the atria into the ventricles. The signal then moved to the Atrioventricular node situated between the atria and ventricles (7).
There was a minimal slowing of the signal here to permit the filling of the ventricles. The signal progressed to the ventricle. From the ventricles, the process of signals, contraction of heart muscle and pumping of blood was repeated. Another signal then started again in the SA node (7). The signals had an organized pattern and elicited the normal waves in the surface ECG. Disorganized electrical signals caused changes in the rhythm. This caused the atria and ventricles to pump at different times. The heart rate simultaneously increased and when it increased to a great deal, the atria began to fibrillate and atrial fibrillation was caused.
The atrial electrophysiology was different from the ventricular one. For the treatment of atrial fibrillation, an antiarrhythmic therapy suitable for atrial fibrillation needed to be instituted (8). The atrium had vessels, nerves and fibrous tissue apart from cardiomyocytes. AF substrate was formed with interstitial fibrous tissue. Fibrosis was a feature seen in hypertension and cardiac failure. The regularity of the triggering of the cardiac impulses was important for a regular heartbeat. The irregularity was what led to the atrial fibrillation; the altered substrate rich in fibrous tissue disturbed the regular impulse formation and thus the irregular reentry (8).
Shortening of the action potential duration triggered the atrial fibrillation through substrate development. The refractoriness was decreased simultaneously. The impulses formed would be increased but of heterogenous nature and conduction became slow (9). These were the electrophysiological changes mainly seen in the atria in AF (7).
Reentry of wavelets was noted in AF whichever class it belonged to. Different mechanisms were involved but finally the multiple wavelet reentry was the common manner of reentry. The maintenance of reentrant arrhythmias was dependent on short wavelength impulse which could be calculated by multiplication of conduction velocity and refractory time (8). When atrial fibrillation occurred, the changes in cell physiology caused the sustenance of the fibrillation.
The process that occurred at cellular level was also defined to fully gauge the electrophysiological basis of atrial fibrillation. Normal atrial cellular physiology needed to be understood initially before going on to the changes in substrate that occurred in heart conditions. This substrate was causing the atria to fibrillate. The cellular changes which contributed to the substrate were detailed as follows. The various processes in the myocardium were the ionic currents and their alterations, intercellular connections and connexins, the passage of calcium through the cell and changes in the cell metabolism due to genetic reasons (8).
The journey taken by the potassium ions was first explained. K+ was found in greater concentration intracellularly. The resting membrane potential of the atrial myocardium used a chemical force which pushed out the excess positively charged K+ ions along the channels as there was a concentration gradient outwards. The resting intracellular potential became negative. There was a reversal potential too in the form of a current which keeps the K+ ions within the cell. The potassium current was smaller in the atrial cells than in the ventricular cells (8). The sinu-atrial node activation, caused the Na+ channels to open and quickly depolarize the cell producing an action potential. Depolarisation also triggered the entry of Ca2+ ions through the specific channels into the cell. The action potential became a plateau.
The next step was the release of Ca2+ ions from the intracellular Ca2+ stores in the sarcoplasmic reticulum and this resulted in myocardial contraction. The ionic currents also went through phases to return the cellular membrane potential and restored the repolarized state of the atrial cell and its excitability (8). The potassium currents which restored the repolarization of the cells of the atrium constituted the most significant current.
Connexins, especially the Connexins 40 and 43 were the channel proteins which allowed the depolarization to travel from cell to cell in the atrium. The connexin which was exclusive to the atrium was Cx40. The depolarization was continued along the sodium channels with the help of membrane proteins called ion channel alpha subunits. The alpha subunits united together to enable the passage of current through the membrane pores (8).
When working together with beta subunits, a change occurred in the biophysical properties of the alpha subunits and the cell activity was directed towards the apt site. The refractory time depended on the action potential duration (APD); it was the duration of time from the first depolarization to the time of restoration of excitability (8). The influence of the APD on the time was related to the formation of the substrate which triggered atrial fibrillation. Cardiac diseases could alter the refractoriness and the conduction velocity of the atrial muscle.
Over the past fifty years, electrical, contractile and structural remodeling had been found to be contributing to the disease process in AF. Improved and better treatment modalities had been found and accepted. Ion channel blockers had been given as therapy (8). Electric remodelling referred to the changes in ionic currents and excitability in the atria (8). Structural remodeling referred to the changes in the constitution of the connective tissue, cell size, glycogen deposition, myolysis, changes in the mitochondria and redistribution of chromatin (8). Contractile remodeling involved the modification of the cell due to the changes in the movement of the Calcium ions and the consequence was the dysfunctional cellular contraction.
Research
Electrophysiological study of dysrhythmias in AF patients had involved the recording of the series of events before programmed electric stimulation and after it. The patient’s response to the stimulation had been analysed systematically to reach intelligent conclusions (1). The AV conduction interval was a significant entity in the research and the researcher had to be familiar with the procedure. Activation mapping was another entity which required the researcher to be equipped. The method of recording the responses was the most significant portion (1). AV conduction defects were recorded from the Bundle of His. When the heart was in sinus rhythm, an H-V interval of less than 30msec was significant as it indicated presence of a potential and was interpreted as a pre-excitation (1).
Disorganized electrical signals could occur in some pathologies and when some medicines were used. The result was that the 2 atria beat irregularly and fast and this was atrial fibrillation. The ectopic activity was fast and sustained and the reentry of wavelets resulted in AF (10).
The atrial effective refractory period became shortened and the atrial cells found it difficult to adjust to the heart rate. Any condition that caused a rise in excitability and reduced the refractory period sustained AF. AF could be re-induced by the overload of Calcium. The ionic pumps were modulated to the event. Angiotensin II was also associated with AF re-induction (10). Class I drugs were used to prevent AF action upon the excitability of the atria. Class III drugs inhibited the mechanisms of reentry by lengthening the duration of the action potential and repolarization (10).
Mapping in research
Research showed that AF could be initiated by programmed stimulation (1). However the mechanism for the maintenance or continuation of the AF could only be investigated through mapping during the fibrillation. Two wavelets had been demonstrated, the reentrant wavelets and the multiple wavelets. The reentrant wavelets were of random nature and hooked onto the pectinate muscle (1). The multiple wavelets were generated as rapid, small and single circuits. The activation time for the circuits was longer than the local refractory period and conduction ability (1). Mapping had been done in human beings too; detailed mapping had been done while the patient underwent surgery.
The studies indicated that the arrhythmia of AF needed to be considered three-dimensionally (1). Previously the AF patients selected had surgery for the Wolff-Parkinson-White syndrome while the mapping was done (1). Kirchhof had reported that recently patients with chronic fibrillation and induced atrial fibrillation who were undergoing surgery for coronary by-pass or replacement of valves (open heart surgeries) were studied (1). Haissageurre’s study focused on the activation of the left atria endocardium in AF (1). He used high density mapping electrodes with five splines and 20 electrodes. The details elicited were significant.
Catheter studies
Catheter studies had also been done using the electrophysiological basis (1). They had been significant for some reasons. Action potentials of the monophasic variety were used for the mapping (1). The amplitude of the signals varied as immensely as the duration. The main reason for considering the catheter studies as significant was the definite diastolic intervals that were seen in some action potentials; these corresponded to the fully excitable gaps in the atrial fibrillation. The second was the variability of the organization of activity of the atria while fibrillating. The activity recorded was of two kinds.
One was the wavefronts which appeared smooth and broad while the other was the irregular chaotic wavefronts which looked fragmented (1). The former arose from both the trabeculated atria and the coronary sinus. The latter was projected from the other parts of the heart muscle like the crista terminalis, the inter-atrial septum and pulmonary vein region (1). The surface ECG showed larger fibrillary waves if the activity was well organised. Koning had reported that the activation from the right atrial free wall corresponded to similar looking patterns in detailed intraoperative mapping (1).
The organization of the activity was probably using space constants of electrical activation alongside the catheters. Quantifying the relationship between neighbouring activation patterns, Botteron and Cain had indicated a smaller space constant for chronic atrial fibrillation and a bigger one for acute atrial fibrillation (1). Intra-operative mapping showed highly complex patterns in the chronic atrial fibrillation with its shorter space constants (1).
Intraoperative mapping with bipolar electrodes
The intraoperative mapping was mainly of two techniques. Schuessler et al used a method which provided a broad picture of the atrial activation but it did not have many details (1). The bipolar electrodes over the atria numbered 156; the largest number being on the right atrial terminal crest. The mapping electrodes were lesser in number on the left atrium. Thirteen patients undergoing the Wolff-Parkinson-White surgery were studied by inducing atrial fibrillation. Reentrant circuits with large wavefronts were seen from the right atria; repetitions occurred every 200 msec. The left atrium showed lesser excitation because of the lesser number of electrodes (1).
Intraoperative mapping with unipolar electrodes
The other technique for intraoperative mapping used unipolar electrodes, 240 in number, in the shape of spoons which were placed manually on the right atrium at a distance of 2.2mm. More detailed information was expected but only the right atrium provided information. Konings et al used this technique to study induced AF in 25 patients having surgical division of atrioventricular bypass tracts (1). Three patterns of activation were recorded. Ten patients showed the type 1 pattern which originated from a single wavefront with no conduction delay. Eight patients with the type 2 pattern had a single wavefront with extremely prolonged conduction or two wavefronts which had a block in between.
The type 3 pattern was seen in 7 patients: three or more wavefronts separated by many blocks and exhibiting slow conduction. (1). The disadvantage about this mapping was the frequent change of patterns during the procedure. The fibrillation interval was longest in type 1 and shortest in type 3. The inference was that when the activation patterns were complex, there were bigger changes in the fibrillation intervals in a direct relationship while the conduction velocity was slower in an indirect relationship (1).
High density mapping
Josephson and his colleagues used Koning’s (1984) method to do high density mapping (1). Their patients had organic heart disease and were being subjected to open heart surgery. Eight patients had chronic fibrillation in addition and ten were subjected to pacing-induced fibrillation (1). The conduction block and fibrillary waves were studied. The evidence of random as well as complete reentry was obtained. In both groups it was that found that most of the wavefronts (70%) were from underneath the plaque in their passage to the atrium. The rest were triggered by epicardial breakthroughs. There were more waves per second in the group of chronic fibrillation patients (29.9 waves per sec) while the other group had less (14.6 waves per second).
The chronic fibrillation patients had a Type 3 pattern. Josephson (1) concluded that right atrial activation during the fibrillation in the chronic atrial fibrillation patients produced a more complex response than when activation was done for the patients whose fibrillations were electrically induced. The lines of conduction block were however similar and situated at right angles to the AV groove. Epicardial breakthroughs were seen in both and the pectinate muscles could be the source. The rhythm was to be considered three dimensional (1).
No evidence was found to indicate electrical remodelling. Atrial fibrillation could be sustained by “drivers” (1). These are focal sites where the atrial activity was regular and rapid. Several researches had turned to this subject. Nine patients who had chronic atrial fibrillation and undergoing heart surgery were selected for the epicardial mapping. Type 1 pattern was seen in the left atrium in 7 of the patients with cycle length about 200 msec. The specific areas which were hypothesized to be drivers were the left atrial appendage and the area outside the left pulmonary veins (1).
High density catheter mapping
Electrical activity during the atrial fibrillation could also be studied by the high density catheter mapping of the atrium (1). Three dimensional dominant frequency maps were used to obtain evidence of the focal sites where rapid activation occurred. The sites were found in the atrial wall in the chronic fibrillation patients and within the pulmonary veins in the induced atrial fibrillation patients (11). Ablation at these sites produced one of two reactions: reducing the fibrillation cycle length or abolishing the fibrillation. Many patients did not need to have ablation at these sites; pulmonary vein ablation was sufficient to restore sinus rhythm, thus this study had a limitation (1).
Another limitation was that focal drivers were not found in patients with permanent atrial fibrillation though they were found in patients with paroxysmal and persistent atrial fibrillation. A recent study had found fibrillations originating in the superior vena cava with a longer cycle in the right atrium (1). Though most patients have their focal triggers in the left atrium, the right too had to be considered for initiation and maintenance of fibrillation. Excitable gaps within the atrial fibrillation provided the possibility of capture of the atrium while fibrillation was proceeding. Kirchhof indicated this in a canine model (1).
Risk factors of AF
Age
Age appeared to the biggest predictor of AF (9). Patients above 65 years were the biggest risks for AF and the average age of the population with AF in the US was 75 (9). The reasons for the elderly group being affected may had been many. The atrium like the rest of the body was subject to age-related changes: the presence of a new substrate triggered multiple reentrant wavelets and prolonged the AF. Age-related illnesses like diabetes, hypertension, myocardial infarction, valvular heart disease and congestive heart failure made the patient prone to AF (12).
Gender
Regarding gender, AF was more prevalent in men than women at any age. The incidence and prevalence of AF in the Renfrew-Paisley project was an example of the general picture all over the world (Refer charts I and II). However, interestingly, the actual number of women with AF was equal to or more than men as more women were alive after 75 years in the total population (9). The Framingham Study by Wolf and colleagues revealed some noteworthy results (3).
Age, previous history of myocardial infarction, or other co-morbidities were the variables adjusted. The development of AF in the Framingham Study was seen rising as aging occurred just like the Renfrew/Paisley study and other UK studies (3). The prevalence of AF became twice in number for the men during the study period from 1968 to 1989 (3). The women however did not show this rising prevalence. The odds ratio was 2.1 for men and 2.2 for women.
This specificity of gender was reported from many other hospitals in the US (13). The odds ratios for diabetics with AF were 1.4 for men and 1.6 for women. However hypertensive men with AF had the bigger odds ratio of 1.5 while that for women was 1.4 (3). Valvular disease with AF showed the highest odds ratio for women at 3.4 while that of men was 1.8. Heart failure and strokes also were associated with AF and simultaneously complicate it. The odds ratio for mortality was 1.5 for men and 1.9 for the women. A note-worthy finding was that most of the mortality in AF occurred immediately after the diagnosis (3). A prevention management in this phase could reduce mortality.
Table 1 showing the odds ratio gender wise for various illnesses.
Information adopted from the Framingham study in the National Collaborating Centre for Chronic Conditions Guidelines (3)
Race or ethnicity
Race or ethnic variations were vague as more studies had been in the western populations. Caucasians were more affected than the African Americans. The number of whites affected was more than twice the number of African Americans by the findings of the Cardiovascular Health Study (9). This picture had also been found in other studies (9). Asians were less affected by AF and ischaemic strokes (9).
Cardiovascular illnesses
Cardiovascular illnesses were also risks for the atrial fibrillation. Researchers had included hypertension, ischaemic heart disease, congestive cardiac failure and valvular heart disease (9). The AF that developed following hypertension was the most common one that was treatable (14).
Other factors
Over the years, the efforts to reduce the incidence of rheumatic fever had been successful; the incidence of valvular disease also had simultaneously reduced. The prevalence of AF with valvular heart disease was now much less (9). Researchers had co-related diabetes mellitus with AF (9). The size of the left atrium and hypertrophy and dysfunction of the left ventricle were also risk factors for AF (9)
Noncardiovascular risks
Factors which were noncardiovascular that posed risks for development of AF included obstructive sleep apnoea (62). Auer had related hyperthyroidism to AF (9). C- reactive proteins had been found to affect the development of AF (15). Personality traits of anger and hostility in men had also been found to lead to AF (16). Genetic reasons had been evaluated recently (17).
One reason for the above discussed risk factors causing a prevalent increase of AF was probably the rise in incidence of the conditions. Another reason could be that these patients were surviving with the AF for longer periods due to better management (9). It could also be a combination of the two reasons. So when we realize that the population with AF is rising, the thought as to whether it is due to enhancement of incidence or improved survival raises a question..
Comorbidities
AF is almost always had associations or comorbidities. Cardiac causes could be ischaemic, rheumatic heart disease, hypertension, sick sinus syndrome or pre-excitation syndromes like Wolff-Parkinson-White syndrome (3). Non- cardiac causes included infections like pneumonia, electrolyte imbalance, cancer of the lung, pathology inside the thorax, pulmonary embolism and thyrotoxicosis.
Surgery
AF had also been found following major surgery like thoracotomy and coronary bypass (3). The appearance of AF produced problems like prolonged hospital stay and the possibility of heart failure. The patients could also go in for strokes or thromboembolism. All these risks automatically enhanced the costs involved. AF could appear in the postoperative period not only due to the type of surgery but also because of the age of the person involved, his physiology and the electrolyte imbalance (3).
Diet and lifestyle
Diet and lifestyle had been known to cause AF. Alcohol or caffeine consumption was believed to lead to AF. A large quantity of alcohol, if consumed within a short period, posed great harm to the heart (3). Alcoholism also worsened the already present AF and put the cardioverted patient in a precarious state. Likewise emotional and physical stress also was related to AF. In patients younger than 65 years, alcohol was found to be a causative factor for AF in 63%. The effects could also be enhanced when these stress factors were combined with other associated illnesses.
Lone AF
Lone AF, a risk factor, was diagnosed when the AF appeared to be not related to any heart disease or hypertension (3). Physical examination did not reveal any abnormality. Chest X-ray did not have any untoward findings. The ECG did not have features of AF but otherwise there were no suspicious features pointing to another associated illness like myocardial infarction or left ventricular hypertrophy (3). Echocardiogram did not show valvular disease or ventricular hypertrophy.
Consequences of AF
Risk of mortality increased when AF occurred in a heart in which function had already been suffering; cardiac output was reduced by about 10%-20%. Ventricular rates if very fast terminated in heart failure or produced fatal cardiac ischaemia (3).
The risk of thrombogenesis was increased in AF. A prothrombotic state was evident by the stasis of blood in the atria, structural changes in the heart, abnormalities of blood vessels and abnormal platelet formation (3). Along with haemostasis, all these factors proceeded to thrombogenesis. This situation in an AF patient was five times more risky than in a patient without the AF and could lead to thromboembolism and stroke.
More disability in stroke patients was seen when the patient had AF simultaneously; the combination of stroke and fibrillation was life threatening (18). The second commonest cause of death following ischaemic heart disease was stroke: developed countries had 4.38 million deaths and developing countries had nearly 3 million of them (18). Industrialised countries reported that 10-12 % of deaths were due to stroke. In the elderly person, AF was considered a risk factor for stroke so therapy with antiarrhythmics in AF was essential to prevent the consequence of stroke. The AFFIRM study confirmed that the antiarrhythmic drugs prevented the recurrence of AF but had no effect on established stroke (18).
It was reported in a meta-analysis that patients who received rhythm control were not so much burdened with stroke. AF patients who received oral anticoagulant therapy had less incidence of stroke: 1.4%. Prevention of stroke involved treatment of the comorbidities of AF like hypertension, increased cholesterol, diabetes and heart failure. The prevention was not easily delivered as several conditions had to be treated in order to achieve prevention (18). The quality of life in AF patients was poor even if they were asymptomatic; only those patients who returned to sinus rhythm had the quality improved (19).
The stroke patient had more chances of remaining in hospital for a longer period. In the age group 50-59, the risk of developing stroke in AF patients, was 1.5% while in the age group 80-89, the risk was 23.5% (3). Age wise, 90% of stroke occurred above 65. AF was believed to cause death in 90% of patients above 65 years of age. Exercise tolerance was diminished and cognitive function was disturbed too in patients with AF (3). Anter and Callans (2) described atrial fibrillation as “the single most important cause of ischaemic stroke”. The AF patients who developed stroke came to about 5% a year, almost seven times more than those who had stroke exclusively without AF. Warfarin therapy was effective for stroke but the risk of life term invalidity and episodes of bleeding still stood.
Classification
AF needed to be classified appropriately as this had not been done even after research for more than 100 years and consequently much had not changed in the manner of therapy of such cases (4). Electrophysiological and clinical classifications had been described by researchers. Newer methods of managing AF through pharmacological and non-pharmacological means had been suggested (4). Attempts were being made to evolve a substance that could be beneficial to the electrophysiological mechanism of the heart but success had not been seen yet (4). The management of AF had thus not been successful and this could be mainly due to the poor classification of atrial fibrillation.
The result of AF having been projected as a single entity had led to researchers adhering to this while investigating approaches to better management. However researchers had started identifying different entities of AF. The future could hold promise in reaching the target of management by which AF will be converted and sinus rhythm instituted and maintained (4).
Electrophysiological classification
Electrophysiological classification had the disadvantage of not assisting clinicians in their practice and management of AF. Only the focal AF arising from the pulmonary veins region obtained any benefit out of an ECG. Among the many methods in ECG, focal and linear ablations had thrown more light on the mechanism of AF but there is still more to know. An early electrophysiological classification depended on the ECG ‘f ‘waves which represented the fibrillatory waves in the 1960s; they could be coarse or fine or flutter type (4). The coarse waves converted to fine ones during carotid sinus massage and just before the sinus rhythm returned. Wells used intracardiac biatrial recordings and Konings improved it further by doing high density endocardial mapping of the right atrium (4).
An intimacy existed between the electrocardiographic findings and mechanisms in the electrophysiological system. Konings’ method was improved by Saksena who did the mapping in both atria simultaneously (4). Then anti-tachycardia pacing which was a non-pharmacological method was introduced. The pacing halted the atrial activity and prevented the progress of the situation. The next discovery was a major breakthrough: the finding of the excitable gap and the use of the critical mass theory to limit the AF (4). The next one was even better: focal AF was identified in the area of the pulmonary veins and radiofrequency ablation could influence it (4). The several mechanisms were the basis of the classifications at different points of time.
Clinical classification had produced more controversy over the years than the electrophysiological one. The differences in the aetiologies, clinical presentations, risk factors, triggers, onset or behavior of illness and effects and responses of treatment had provided cause for wide discussion (4). An accepted clinical classification was a recent one by the American College of Cardiology, American Heart Association and the European Society of Cardiology (20).
Primary AF is classified as follows.
First detected episode –Symptomatic
- Asymptomatic
- Of unknown beginning
Recurrent
- Paroxysmal when the AF terminates spontaneously
- Persistent when it is sustained
Permanent or accepted AF
The first detected episode could be self-limiting or remained symptomatic or the onset could be unknown (3). This episode could be a singular one or become recurrent. Therapy was instituted only if the symptoms were severe. After having 2 or more than two episodes the patient was deemed to have recurrent AF. If the episode ended spontaneously it was classified as paroxysmal. This paroxysmal type usually spontaneously ended by itself within 24 hours or lasted for more than 7 days but was self-terminating. Therapy required the prevention of recurrences to control the heart by using medicines and anticoagulant if necessary.
AF was named persistent if it is sustained even after therapy turned out to be successful; the usual therapy included rate control and anticoagulants and cardioversion if required. The persistent variety lasted for more than 7 days where cardioversion had not yet been attempted or failed when attempted (3). The first detected episode could turn out to be persistent. Several occasions of recurrent paroxysmal AF could result in the recurrent persistent AF (5). Cases of AF extending beyond one year without cardioversion were termed permanent accepted.
Secondary AF was associated with acute ischaemic disease of the heart and with cardiac surgery. Infections of the coverings of the heart like pericarditis and myocarditis were also associated with AF (5). Pulmonary causes were embolism, pneumonia and acute pulmonary illness. However the AF disappeared as soon as these associated conditions were treated.
Epidemiology
Padanilam (21) had described the prevalence of AF as a cardiovascular epidemic while others had described it as a major global health problem. The population which was higher in the aging group and the incidence of cardiac illnesses had contributed to the rising fatalities; however these aetiologies could not explain the complete picture (21). It was right to contend that the aging process was definitely a risk factor as about ten percent of the elderly above 80 years were affected.
More information needed to be elicited regarding age, gender, race and cardiovascular disease (21). Reasons were unknown of AF for the rise of the incidence and prevalence (21). Children were not known to have AF unless they had congenital problems (3). Hospital discharges were evaluated in the US. An analysis of 3806000 discharges of patients indicated that 1.5% of them had diagnoses of arrhythmias (22). AF was the arrhythmia in 35 % of them.
Aetiology
The obvious aetiologic factors were the increase in the elderly and cardiac illnesses. The paroxysmal, persistent and permanent types had been distinguished at the international level by the different heart societies: American College of Cardiology, American Heart Society and the European Society of Cardiology (3). However these distinctions were not made when epidemiological studies were done.
There had been studies which indicated that AF could not after all be a feature among the elderly (3). The decrease in the number of rheumatic fever and the rise in the elderly population both had contributed to the changing epidemiology over the years. The main aetiologies in the modern world were hypertension and coronary artery disease (22). Lone AF referred to the AF seen in people under the age of 60 and who had no obvious cardiac disease. It had an incidence of 31% but some of these patients had associated heart disease. In another study, the incidence was 12 % (22). Out of 6 strokes, one was accompanied by AF.
Statistics
Approximately 2.2 million people were affected in the United States but this figure could not have included the asymptomatic individuals of which there are many. A 2.5 fold increase was expected by 2050 coming to about 20% of the population (21). The Population Projections Program of the US Census Bureau had estimated that the number of people above 65 years would be 82 million by 2050 (23). This was expected to cause a rise in the number of AF patients by 2.5 times (24). The possibility that population studies could not be right in their estimate of the AF-affected people must not be forgotten.
The Renfrew/Paisley study in the UK had 100 cases documented with AF in the age group 45-64 (See Bar Charts I and II, pg. 25). The incidence definitely increased with age and there were more men affected, 53 against 47 women. It was noted to be 0.54% per 1000 person-years. The prevalence was 2.4% in the West Birmingham project. However the incidence among Indo-Asians was only 0.6%. The general practice in a Newcastle survey showed a prevalence of 4.7% in the age group above 65 years. Acute medical admissions consisted of 3-6% of AF (3).
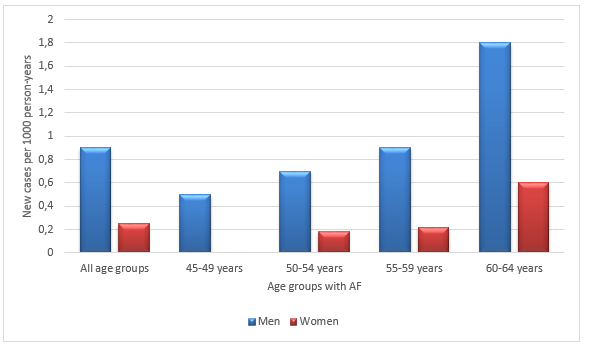
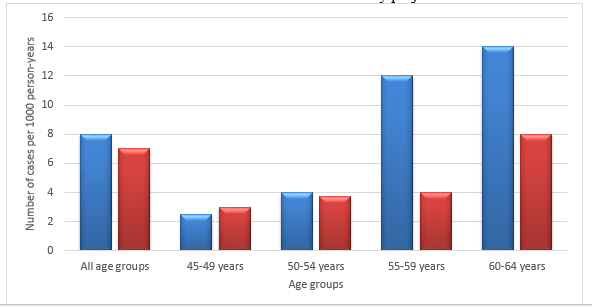
Bar Charts 1 and 2 are both adopted from National Collaborating Centre for Chronic Conditions Guidelines (3)
In patients who had non valvular AF, the increase in brain ischaemia was more than 7% (9). In those who had rheumatic heart disease, the increase in risk for stroke was 17 times more when matched with the Framingham study for age (9). The risk of stroke in AF increased by two times in the Manitoba Follow-up study when other risk factors were not considered (9). Age had a direct relationship with the risk of stroke: in the age group below sixty, the risk of stroke was 1.5% while the risk became 23.5% in the age group above eighty (3).
The Framingham study provided significant information about epidemiology of AF. People in the age range of 28 to 62 and free of cardiac illness (5209 of them) were selected for the study (9). The follow-up continued for 38 years; AF had developed in 264 men and 298 women of the 2090 men and 2641 women (9). A rising prevalence was noticed; the prevalence was 0.5 % in the age group 50-60 while it was 8.8% for those above 80 (9).
A similar result was seen in the Cardiovascular Health Study which had 5201 men and women as participants for five years (9). The Rochester Minnesota study was another large population-based study (9). Other researchers, Feinberg et al found that 2.23 million US people had AF (9). The even larger population study from the West of Scotland had results similar to the Framingham study; the prevalence was 0.65% in the age group 45-65 (9). The ATRIA study which investigated the anticoagulation and risk factors for people above 20 years in a population indicated that the prevalence was 0.95% (Go AS et al). Interestingly another study indicated a prevalence of 0.9% for people younger than 55 and 9% for those above 80 (24).
Management
Management of AF involved the control of rate and rhythm, reducing the risk of thromboembolism and the treatment of associated or comorbid conditions (10). Rate control could be achieved by suppressing the action of the calcium currents or by administration of beta blockers or digitalis drugs (10). Non pharmacological methods and electrical cardioversion were also practiced. Only a few drugs, Class Ic agents and amiodarone, were available for rhythm control. However their disdvantages were that they had many side effects and were unable to reduce mortality.
On seeing an AF patient the first decision was to choose between rate control and rhythm control to be instituted and assess the stroke risk possible. The repolarization time needed to be prolonged for maintaining sinus rhythm. The best drug should be able to abolish AF and maintain sinus rhythm (25). The AFFIRM (Atrial Fibrillation Follow-up Investigation of Rhythm Management) clinical trial indicated that a return to sinus rhythm and its maintenance reduced the risk of death. A similar opinion was provided in the DIAMOND study (26). The AFFIRM trial indicated rate control in older patients.
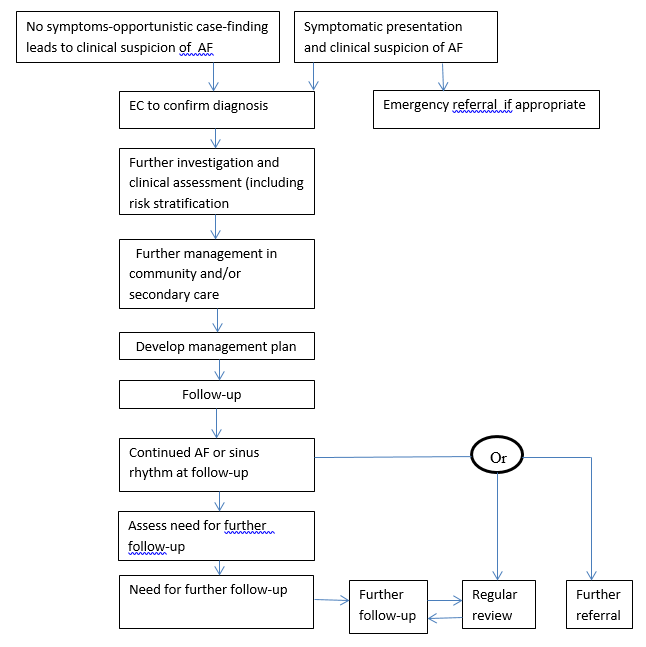
However the study found no significant difference in the treatment with rate and rhythm controls (27). The study had mostly older patients who were asymptomatic. Drug toxicity was ruled out. AFFIRM suggested that the maintenance of sinus rhythm with antiarrhythmics was important and beneficial but increased mortality due to drug toxicity cancelled the benefit. Therapy for AF was possible but the long drawn out life-time illness could cause harm in the form of side effects. Timely cardioversion was the appropriate therapy for atrial fibrillation but if the remaining therapy for rhythm to bring it to a sinus one was improper, the efforts at cardioversion became a vain one (27). The AF care pathway flowchart shown in the NCC-CC Guidelines provided an effective guidance to clinicians (See following page).
Adopted from National Collaborating Centre for Chronic Conditions Guidelines (3).
Guidelines
The NCC-CC guidelines of 2006 specified the drugs that could be used in cardiac diseases. The drug therapy initiation was to be under the clinician’s supervision. Class 1A drugs by the Vaughn-Williams classification were to be used in hospital for increasing the duration of the repolarization and the QT interval but these drugs were no longer available (27). Lone AF patients only were recommended Flecainide and propafenone.
Patients having structural heart disease and heart failure were given the Class III antiarrhythmics like sotalol and dofetilide in hospital initially (27). The kidneys were involved in the clearing of these drugs and so the drugs had to be selected with caution. For patients who had a structural heart disease along with a kidney problem, or a long QT interval, amiodarone was the only choice (27). Dronedarone had been approved by the Food and Drug Administration for use in AF. This drug belonged to Class III.
Treatment strategy
While treating atrial fibrillation patients, symptoms were to be controlled and sinus rhythm restored, embolism prevented and heart failure managed. Embolism produced stroke. The flow chart on page 28, adopted from the NCC-CC Guidelines of 2006, provided an idea of how to decide whether rate control or rhythm control was to be implemented for management. The diagnosis of paroxysmal AF or persistent or permanent had to be made initially from the history, clinical assessment and investigations. Paroxysmal AF required rhythm control, permanent AF needed rate control and persistent AF could have either one. Warfarin was used as the anticoagulant of choice except for patients with seizures, gait problems and syncope (25).
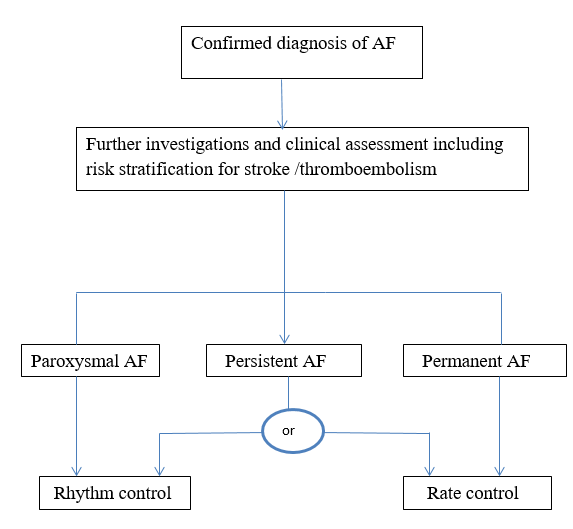
Adopted from National Collaborating Centre for Chronic Conditions. Atrial fibrillation: national clinical guideline for management in primary and secondary care. London Royal College of Physicians.
Recent approaches
The recent approaches included the use of pacemakers or defibrillators. Catheter ablation techniques were another novel approach. Elderly patients who did not have contraindications were selected for linear ablation and pulmonary vein ablation. Patients who could not have drug therapy for atrial fibrillation for rate or rhythm control had the ablation methods. Focal AF could be managed with radiofrequency ablation (4).
Required characteristics of Antiarrhythmic drugs
The repolarization time needed to be prolonged for maintaining sinus rhythm; the best drug could abolish AF and maintain sinus rhythm (25). Without damaging the heart, the drug was to block the ion channels. The efficacy of the pro-arrhythmic drug was based on the change in heart rate. Sotalol and amiodarone had emerged as the best drugs. If the AF relapsed, the anti-adrenergic actions of these drugs proved beneficial. Flecainide and propafenone had specific actions on the electrophysiological mechanisms of the atria. Their newer generations of dronedarone, tedisamil and RSD 1235, all had variations in their atrial selectivity (25). Anti-fibrillatory drugs in the future will convert and maintain sinus rhythm without producing toxic side effects.
Rhythm versus rate control
Rate control was obtained by the action of drugs that suppressed the calcium currents and the use of beta blockers or digitalis. Rhythm control was the return of the heart to normal sinus rhythm. In the comparison of rhythm and rate control, mortality was not affected by rhythm control; the emphasis was to be on the rate control (28). The AF-CHF trial indicated that even in heart failure, the attempt to control rhythm was not beneficial when compared to the rate control even though theoretically, one would have thought otherwise (29).
The DIAMOND study (Danish Investigations of Arrhythmia and Mortality on Dofetilide) consisted of several trials all for assessment of the usage of Dofetilide for treating AF, its efficacy and safety. These studies indicated that the maintenance of sinus rhythm was more significant for prognosis whether anti-arrhythmic drugs were administered or not (2). The patients who had sustained AF had poor prognosis. More than the treatment strategy, it was the maintenance of sinus rhythm that led to a good outcome.
The AFFIRM trial echoed the Diamond study. However neither study could actually indicate whether it was sinus rhythm which actually directly affected the survival rate of AF patients or whether it was because the sinus rhythm changed the course of events directly or affected other associated illnesses. Overinterpretation and thoughtless application had often affected results (2). Dronedarone was used for both rate control and rhythm control (10).
Pharmacological treatment drawbacks
In spite of so many advances in the field of Medicine, the pathophysiology of AF was yet to be fully understood. The ion channel blockers that were used in therapy were not totally successful in terms of safety or efficacy due to this drawback. Following the absence of symptoms after drugs in studies, it was not proper to assume that this absence of symptoms of AF occurred just because of the drug used. An absolute explanation was not possible.
The question as to whether rate control or rhythm control was to be first achieved in the therapy of an AF patient was still debated (2). Whichever therapy was selected, the rates of mortality were worrying till now. Newly diagnosed patients with AF were provided modern treatment and followed-up for 21 years; this longitudinal cohort study did not change the statistics for mortality associated with age or gender after adjusting for comorbidities (30). It is obvious that we have to go a long way if we are to make any indent into the mortality statistics where AF is concerned. It is of no point to simply join the flock which is concentrating on pharmacological treatment. New therapies have to be found to ensure the survival of AF patients (2).
Comorbidities
The co-morbidities of Hypertension, diabetes mellitus, obesity, valvular and non-valvular heart disease even after adjustment, ended in a poor prognosis (31). These had to be treated simultaneously with AF. In this modern age when sufficient improvement had occurred for management of coronary heart disease and heart failure with reduction in mortality, AF management was far behind. With anti-arrhythmic drugs, only symptomatic treatment was provided; survival was not ensured (2). The therapies for heart failure had become improved with the discovery of the neuroadrenergic activation. Just as heart failure had been conquered, AF management also must be improved by scrutinising the mechanisms involved in the production of AF (2).
Quality of life
Undetected AF blocked the treatment for some elderly patients at risk of stroke. The asymptomatic patients who underwent successful cardioversion usually felt much better (2). The quality of life is poor in patients with AF when compared to patients who do not have AF, matching for age. With improved quality and regular exercise performance, sinus rhythm was restored. The Canadian Trial of Atrial Fibrillation (CTAF) was a study of the effect of AF on quality of life (2).
They concluded that the quality of life was definitely lower in patients with AF when compared to healthy controls. Young patients were involved in the study; the study had more significance in that the incidence of co-morbidities was low. The AF itself was the reason for the impairment of quality of life (2). The patients were in addition receiving medicines to control the ventricular response. They had the difficulties imposed by the AF and the feeling of general well- being was diminished.
The impairment was affecting their normal physical functioning, social life and vitality. However the restored sinus rhythm produced an upheaval towards improved functioning all around. Symptoms were simultaneously reduced. The implication that these results could have occurred due to rate controlling methods was proved otherwise by the comparison of patients who had recurrences and those who did not (2).
Therapy for new cases
The risk of mortality was higher in the patients with new AF. The therapeutic considerations for such patients were significant due to the pathophysiological importance. Miyasaka studied AF mortality trends for 21 years and found that the risk was higher in the 4 months just after diagnosis. The hazard ratio was 9.62 while it was reduced to 1.66 after the 4 months. (30). The COMET (Carvedilol or Metoprolol European Trial) and the Framingham study both had a similar analytic finding. It could be that pathophysiological mechanisms were set in motion for the left atrial remodeling. The change from paroxysmal to persistent AF was promoted alongside (2).
The prothrombotic state was also a part of the new-onset AF. Platelets were activated and plasminogen activation inhibitor was increased. The endocardial nitric oxide synthetase was decreased in expression (2). These alterations were seen slightly before the AF is symptomatic. Therapy with anti-arrhythmic drugs tended to produce harmful effects during the initial phases. Warfarin therapy was risky for the elderly even if strictly adhering to the protocol (2). Early cardioversion needed to be done to interrupt the vicious progression from paroxysmal to persistent AF. Patients had been known to continue without antiarrhythmic drugs after the cardioversion if any triggering factors were identified and treated promptly.
The AFFIRM study indicated that the beneficial effects of sinus rhythm were abolished by the harm of antiarrhythmic drugs now available (22). The Atrial Fibrillation Follow-Up Investigation (AFFIRM study) was a significant study for AF (22). A large multicenter was used for the setting. Anti-arrhythmic drugs were used to study the comparison of rate control to rhythm control patients who just had AF. Anticoagulation therapy was also given.
There was no difference in the mortality or incidence of stroke. The inference was that any of the two approaches would work equally well as a first line of approach for new cases. Patients with AF required rate control and anticoagulant therapy. Researchers had begun to believe that it was unnecessary to have sinus rhythm restored: the AFFIRM and the RACE studies reported that aggressive return to sinus rhythm was not needed in many patients (42). However cardioversion was required in symptomatic patients. Many patients had their arrhythmia disappearing when their precipitating cause was treated. Direct current cardioversion was an option for therapy in AF (42)
Dronedarone
Dronedarone was a noniodinised analogue of amiodarone but it did not have the adverse effects of amiodarone (18). The iodine portion was replaced by a methane-sulphonyl group (27). Dronedarone had been advocated for treatment of atrial flutter and atrial fibrillation (18). It had a benzofuran derivative and belonged to Class III antiarrhythmic drugs though it possessed the features of all the properties coming under all four classes of the Vaughan-Williams classification (18). The electrophysiological properties of multichannel blocking were similar to amiodarone though structural differences were present (27).
Christiansen and his colleagues undertook a review of 7 studies: DAFNE, ADONIS, EURIDIS, ATHENA, ANDROMEDA, ERATO and DIONYSOS. DAFNE was the only study focusing on dose-finding. Maintenance of sinus rhythm was the idea behind 3 studies ADONIS, EURIDIS and DIONYSOS. ERATO was concerned with rate control. ATHENA and ANDROMEDA (Antiarrhythmic trial with DROnedarone in Moderate to severe congestive heart failure Evaluating morbidity DecreAse) investigated mortality and morbidity (18).
DAFNE (Dronedarone Atrial FibrillatioN study after Electrical cardioversion) established the dose of dronedarone as 400mg in twice daily dosage. The dosage was decided after the DAFNE trial where 3 dosages were given twice daily. The most efficacy was found with the smallest dosage of 400 mg.(27). This was also the dosage where lesser gastrointestinal side-effects were seen. This dosage was repeated in EURIDIS and ADONIS trials. Both these studies showed a larger interval to the first recurrence of AF and a reduction in the ventricular response in the recurrences.
Dronedarone was suggested to be of a superior action to a placebo in the maintenance of sinus rhythm in EURIDIS (EURopean trial In atrial fibrillation or flutter patients receiving Dronedarone for the maintenance of Sinus rhythm) and ADONIS (American-Australian-African trial with DronedarONe In atrial fibrillation or flutter patients with Dronedarone for the maintenance of Sinus rhythm).
However in DIONYSOS, the dronedarone was found to be less efficient than amiodarone for the reduction of mortality mostly due to congestive heart failure. (18). The study was not continued. Dronedarone could have been interfering with the heart failure medicines by acting on the angiotensin converting enzyme inhibitors. ERATO “(Efficacy and safety of dRonedArone for the cOntrol of ventricular rate during atrial fibrillation)”concluded that dronedarone decreased the ventricular rate in chronic AF (18). The increased mortality of patients with heart failure led to the discontinuation of the ANDROMEDA study.
The ATHENA “(A placebo-controlled dronedarone double-blind parallel arm Trial to assess the efficacy of 400mg twice daily for the prevention of cardiovascular Hospitalisation or death from any cause in patiENts with Atrial fibrillation/atrial flutter)” provided a better conclusion (18). It showed a reduction in hospitalisation and mortality for cardiovascular patients (18). A post analysis indicated that 54 cases of strokes were seen when treatment was initiated with Dronedarone (18). In comparison, 76 strokes were seen among patients on placebo. Christiansen concluded that sinus rhythm could be maintained by Dronedarone and stroke reduced (18).
The risk of stroke per year was 1.2% in the dronedarone group and 1.8% in the placebo group as reported by Wolbrette et al (27).The data did not reveal if the result was due to the alteration in the AF or if dronedarone had other effects in Chritiansen’s study (2010). Deaths had been divided into four groups. The cardiac cases were grouped into arrhythmic and nonarrhythmic. There were two more groups , the vascular and non vascular (18). This information was collected from hospital reports mainly. Death reports were also scrutinised for information. The Adverse event reports which contained the words “stroke, cerebrovascular accident and cerebellar haemorrhage” were also investigated.
The number of stroke patients who were not hospitalized or died of stroke was noted under adverse events. Ischaemic strokes that had occurred in the dronedarone group were 33 out of 46 and in the placebo group, 49 out of 70. Haemorrhagic strokes were equal in number in both groups (18). Side effects were “nausea, diarrhea, bradycardia, rash, raised serum creatinine and QT prolongation” (27). Dronedarone could be assumed to have reduced the risk of stroke through the moderate reduction of blood pressure and decrease in the heart rate (27).
Dosage, action and metabolism
Dronedarone was administered as 400 mg twice a day. Absorption was assisted if taken with food and increased 2-3 times. Plasma concentration of the drug became steady by 5 days. Half-life was about 24 hours. The initial dosage was given in the outpatient department. Caution was to be practiced when selecting other liver-metabolized drugs to be given with Dronedarone as it inhibited the systems of CYP3A4 and CYP2D6 both coming under the CYP450 system through which Dronedarone was metabolized (27).
This is why Digoxin or Simvastatin could not be used with Dronedarone. Other cardiovascular drugs like diltiazem (beta blocker) and verapramil (calcium channel blocker) also could not be used safely with Dronedarone (27). If care was not taken, bradycardia or toxicity could result in more harm than good. Generally the interactions were lesser than amiodarone. Dronedarone did not interact with Warfarin. It also had a tendency to raise serum creatinine levels even when glomerular filtration was not changed.
As the dronedarone was a noniodinised amiodarone analogue, adverse effects were believed to be less. Dronedarone had properties which are antiadrenergic. It was also inhibitive for transmembrane potassium currents: the different rectifier currents and the transient outward current (18, 27). The sodium and L-type calcium channels were also blocked. Considering the potency for action on the K+ current (through acetyl choline receptor) when compared to amiodarone and sotalol, dronedarone was 100 times better. The effect in vitro also indicated that the largest sodium currents and the potassium currents were both better inhibited by dronedarone than amiodarone (27).
Amiodarone was known to produce skin discolouration and untoward effects on the “lung, liver and thyroid” (18). The absence of iodine in dronedarone had done away with these ill effects of thyroid and pulmonary toxicity (27). The methane-sulphonyl group had made dronedarone less lipophilic; it also had a lesser half- life. These made dronedarone better accepted (27). It could not be used in patients with digoxin therapy but created no disturbance in Warfarin metabolism (18). Like most medicines, it was metabolised in the liver and excreted via the faeces. Animal studies have shown that dronedarone was terratogenic (27).
Dronedarone, a Class II antiarrhythmic drug, had properties which covered all the four classes of Vaughan Williams antiarrhythmic drug classification (10). Like Class I drugs, it was able to inhibit the rapid sodium current. Its inhibitory action on the alpha adrenergic and beta adrenergic receptors was a property of Class II. The primary mechanism of action was the blocking of the outward potassium currents, the action belonging to Class III.
It also blocked the slow calcium inward currents. The Class I and Class III effects led to rhythm control through the increase in the refractory period and the slowing of cardiac conduction. Its inhibition of proarrhythmic effects could be acting through the decrease in the repolarization caused by regular blocking of the outward currents (10). Class II and IV effects contribute to the rate control properties. The antiadrenergic properties also are features of Class II while the blood pressure lowering property is of Class IV.
Indications
The indications for dronedarone by the FDA approval are paroxysmal or persistent AF in patients who were in sinus rhythm or who were to be cardioverted (18). Additional risk factors were as follows: age more than 70 years, diabetics, hypertensives, prior strokes, bigger left atrium, and a Left Ventricular Ejection Fraction lesser than 40 %. The earlier ANDROMEDA study had put this LVEF at less than 35% (27).
The ATHENA participants were selected using these criteria. However the ATHENA study has not provided information as to whether the dronedarone could be used for permanent AF (18). The question remained as to whether dronedarone could be used for patients who needed rate control as the studies mentioned had been for rhythm control. The ERATO trial which investigated rate control showed a positive result for the use of dronedarone in reducing heart rate. The ATHENA trial could be interpreted as implying that dronedarone could be used mainly for rhythm control. However should patients on dronedarone develop permanent AF, it may not be necessary to stop the drug (18).
Contraindications
Dronedarone was contra-indicated in Class IV heart failure (27). Class II-III and IV patients were not given this drug if their hearts were recently decompensated and needed hospitalization or referral. Patients with Atrioventricular block or sinus nodal dysfunction without a pacemaker could not have dronedarone. When medicines like ketoconazole, itraconazole, voriconazole cyclosporine, telithromycin, clarithromycin, nefazodone or ritonavir were being used, it was better to avoid dronedarone as these medicines were major CYP3A inhibitors (27). Dronedarone is also not used in patients who were taking QT prolonging drugs or herbals.
Those who had a corrected QT interval >500msecs. or PR interval of >280msecs, could not be given dronedarone. Hepatic dysfunction was another contraindication. Pregnant women or ladies who planned to become pregnant had to avoid this drug. Patients who had been newly diagnosed with heart failure or worsening heart failure, hypokalaemia or hypomagnessemia or corrected QT interval, could be given dronedarone with plenty of caution (27).
Stroke
The dronedarone group exhibited an incidence of 0.3% and the placebo group had an incidence of 0.5% in the EURIDIS study. ADONIS had a similar result (18). In ANDROMEDA, 1.3% of the dronedarone group of patients developed stroke while 0.9% of the placebo group also did so. The information on the risk of stroke was however adopted from the ATHENA trial. Dronedarone had an action of suppressing AF but details were not available from ATHENA.
The number of patients who were given dronedarone and had stroke reduced through the study. The percentage of mortality from cardiovascular illnesses and the resulting hospitalisations were reduced. Dronedarone was believed to have influenced the number of strokes through two other beneficial effects: reduction of heart rate and blood pressure (18). Though the changes were minimal, they could have produced changes in patients who already had other risk factors in the study.
The finding of the reduction in stroke was a post hoc finding which was not expected at the time of study. The reasons as to why the antiarrhythmic drugs reduced the stroke were not understood even in the post hoc analysis causing a limitation to the study. Future research should be able to improve quality of life by reducing the incidence of stroke through rhythm control and when this is achieved, the next step would be to treat patients who have higher risk for stroke (18).
Ongoing trials with dronedarone
The PALLAS and the ARTEMIS AF were two ongoing trials for dronedarone (10). In the PALLAS “(Permanent atrial fibrillation outcome Study using Dronedarone on top of standard therapy)” the year 2010 saw the beginning of randomisation in the study (10). Patients having permanent AF and other risk factors in association were focused upon. The end-point was any one of three events: major cardiovascular event under which stroke, embolism, ischaemic heart disease or cardiovascular death were included, first unexpected hospitalization for cardiac causes or death from any cause (10).
The ARTEMIS AF (A Randomized International multicenter open-label study to document optimal timing of initiation of dronedarone: Treatment after conversion with loading dose of amiodarone in patients with persistent atrial fibrillation requiring conversion of AF) also started randomization in 2010 (10). The best regimen for administration of dronedarone after stopping amiodarone was being investigated. Two substudies were involved. Patients with persistent AF needing conversion were selected for the ARTEMIS AF loading trial. The ARTEMIS AF long term trial looked into the problems that were prevalent due to the conversion from amiodarone to dronedarone (10).
Non-pharmacological therapies for atrial fibrillation
The management of arrhythmias using nonpharmacological therapies had become a common event in the near recent (1). Implantable devices were being used for various cardiac problems. Bradyarrhythmias were treated with pacemakers. ICDs or implantable cardioverter defibrillators worked for tachyarrhythmias. Biventricular pacing was for heart failure (J1). The indications for implantable devices had been widely researched and evolved over the years for reduction of mortality and improvement of quality of life. There was speculation that soon these devices could become a first line of treatment for arrhythmias. Electrophysiological studies would soon clearly define the role of the implantable device. There could even be a single device for all cardiac illnesses.
Great advances had been made in the search for implantable devices for bradycardia. The journey from nonprogrammable pacemakers which had no sensing abilities to programmable and sensing devices had been lengthy (1). The modern pacemakers had various advantages; they were programmable to suit the patient’s requirements. Pacing mode underwent change to be accurately responding to the arrhythmia. Overdrive pacing was also possible. The arrhythmias experienced by the patient could be stored for future diagnosis and therapy making them useful diagnostic tools (1). Indications for the pacemaker continued to increase. The implantation of a pacemaker following ablation of the AV node was able to control AF with a fast ventricular response (1).
Radiofrequency catheter ablation
Recurrence of atrial fibrillation had to be prevented as it was fatal (4). New sinus rhythm procedures were evolving. An alternative method to maintain sinus rhythm was the radiofrequency catheter ablation (26). Radiofrequency ablation was a procedure that produced alterations in the left atrium; the beginning of pulmonary vein was another target. Sinus rhythm was maintained in both situations.
Focal ablations were occasionally necessary especially for focal fibrillation. Radiofrequency energy or anatomically-based techniques could be used. Radiofrequency energy and ultra-sound energy could cause destructive lesions. These fairly new procedures had not been studied in detail yet. So indications, success rates and complications were not definite (4). The Maze open-heart surgery procedures which used the catheter had not been studied for efficacy yet.
The trials for ablation had been known to be “more stringent” in the ECG detection than for the trials with the antiarrhythmic drugs ((32). Stabile’s trial demonstrated the achievement of freedom from AF at 1 year; 93% of the catheter ablation patients and 35 % of the antiarrhythmic drug patients achieved freedom from AF. A similar finding was reported by Wazni et al (33) where only 13% of patients with catheter ablation studied had recurrence while 63% patients with antiarrhythmic therapy had recurrence. The quality of life in patients who had catheter ablation was invariably better (2).
Noheria’s meta-analysis (34) showed a recurrence-free survival rate of 75 of 7% with catheter ablation while the antiarrhythmic therapy group had 18.8%. Generalising the results of the various trials was not recommended as the trials had been short and participants were young and fairly healthy.
The trials RAAFT “(Radiofrequency Ablation versus Antiarrhythmic Drugs for Atrial Fibrillation Treatment-35th ref), the CACAF-2 (Catheter Ablation for the Cure of Atrial Fibrillation-36th ref) and the CABANA (Catheter ABlation versus ANtiarrhythmic Drug Therapy for Atrial Fibrillation)” were larger studies with a wider demographic pattern and longer duration of follow-up (34). These could produce more definite results. The role of catheter ablation proved to be clearer. The CABANA had 3000 participants with demography like the AFFIRM study. However the response to catheter ablation was additionally studied in CABANA (34).
Pacemaker for monitoring
Pacing was known to reduce the frequency of fibrillation. Anti-tachycardia pacing was a non-pharmacological method of management. Recurrence could be treated by using low- energy atrial cardioverting shocks through electrodes. Electrophysiological studies served many functions in cardiac therapy. Sinus node dysfunction could be easily detected in patients who were symptomatic but in whom bradyarrhythmias had not been recorded; pacemakers were indicated for these patients (1).
The increased length of the A-V interval, pacing-induced block and the alternating bundle branch block could be documented to indicate the chances of instability of the His Purkinje system and thereby cause a bundle branch block. Studies could also elicit the possibility of A-V block. Retrograde conduction which had significant implications could be revealed. Patients suited for biventricular pacing could also be investigated for the purpose of electrical resynchronization. The pacemaker as a monitoring device was extremely useful (1)
AV nodal ablation
Patients with permanent atrial fibrillation not responding to medical therapy could have AV nodal modification by AV nodal ablation (4). Non pharmacological approaches could soon be subjected to research on a wide scale.
Costs
The management of atrial fibrillation was a significant inclusion in specialized health care as AF produced morbidity, caused a poor quality of life and some amount of mortality (9). A thorough evaluation and management was essential for any new case of atrial fibrillation or an old case already diagnosed. The factors which influenced the selection for the type of management depend on the haemodynamic status, symptomatology and the presence of comorbidities or associated illnesses and the episode duration (9). Investigations included laboratory testing initially. Simultaneously monitoring of the patient was carried out. The cardiac imaging and hospitalization were the next essential things to be done. Incidentally these were the factors which cause the sky-rocketing of the costs.
The mean costs incurred in patients who were discharged from the emergency department were compared to those for hospitalized patients; $1878 and $2012 were the computed figures (9). AF patients took up 9% of the costs of consultation and 8% of the costs for investigation of the total costs for the care of AF patients as indicated in a French survey (9). Attempts had been made to reduce the costs of management of acute AF by Dell’Orfano and his associates (9). The development of clinical guidelines for the practice was the initial procedure adopted to keep in control the costs incurred. They touched the appropriate usage of cardioversion, the less costly drugs and timely referral to AF outpatients in order to reduce hospitalisations. The net result was a reduction of costs to $1400 for one patient.
In spite of the attempts to bring down the hospitalization rates, hospitalization was inevitable for many. Studies had indicated that a good number end up in hospital. Sweden had a study, the Multifactor Primary Prevention Cohort which had a follow-up duration of 25 years. Hospitalisation was required for 10.1% of the patients in the study.
Cardioversion
Introduction
The treatment used for persistent atrial fibrillation was external cardioversion (37). It was believed that “transthoracic external electric cardioversion was the standard method to restore sinus rhythm in patients with persistent atrial fibrillation” (37). However other researchers considered that this was not always successful (37). Kirchhof thought that the future studies should attempt at improving the method of cardioversion for the socio-economic benefit of humankind (37). Cardioversion could be external or internal, using biphasic or monophasic waveforms or using different electrode positions and using different energy levels of shocks.
History
Cardioversion was a major therapeutic advance of the 20th century. The pioneers of cardioversion were “William Kouwenhoven, Claude Beck, Paul Zoll and Carl Wiggers and Lown” (38). The efforts of Dr.Bernard Lown and his associates had evolved this major development which had changed the treatment of cardiac illness (38). Lown presented this at a conference in 1962. The cardiologists responded as in any other new discovery: disbelief being the main reaction. Lown’s animal studies provided evidence of the electrical basis of the rhythm disorders which we now know were caused by reentry circuits which were not in the normal course of events. Lown then developed a direct current capacitor to produce shocks. Safety and efficacy of the procedure were defined in the canine model (38).
Electrophysiological basis
A series of electrical and anatomical changes occurred in the atria with the onset of AF. These changes then initiated further changes so that the AF remains. Issa had commented that once AF started it perpetuated more AF; this truly described the process in AF (39). Paroxysmal AF could progress to become persistent and later permanent. The atria showed remodeling when AF began. With repeated AF, the remodeling became progressive and irreversible and unresponsive to drug therapy and cardioversion (39). The ion channel activities showed alterations. The atria showed partial depolarization and the atrial refractory period became short.
Left atrial remodeling
Left atrial remodelling was the process of adaptation of the atria which regulated changes in the cardiac myocytes. The aim was to maintain homeostasis as a means of tiding over the crisis of stress (2). The changes occurred in the ionic channels, intracellularly, extracellularly and based on the genomic instruction. The removal of factors, which played the role of stressors, reversed the process and the myocytes were undamaged.
If untreated, damage occurred within and outside the cells. A self-propelling chain of reactions was set in motion and the consequences included irreversible damage of the left atrium (2). Electrical remodeling began when the paroxysmal AF progressed to persistent AF. The AF itself promoted alterations in the structure and functions of the cells which favoured the continuation of the AF (2). Studies on the goat’s heart were done using pacemakers. Rapid pacing produced long lasting AF. A higher risk of induction was seen.
The chances of returning to sinus rhythm spontaneously were small. The interpretation was that when the heart beat was fast, the calcium current was directed inwards and caused a calcium overload. This was toxic to the cell. Adaptation was the attempt to diminish the load to protect it. The membrane channels which allowed the Calcium to enter were down-regulated to lesser activity (2). The action potential duration was lessened and this further promoted the continuation of AF which could be the reason for calling the process maladaptive remodelling (2).
The dilatation of the atria and interstitial fibrosis were features of structural remodeling (61). Arrhythmic conditions like atrial fibrillation had fibrosis which was a typical feature. The myocardial parenchyma underwent degeneration due to the frequent fibrillary activity in AF. In the repair process collagen deposits replaced the degenerating parenchyma. Though this atrial fibrosis was seen in a number of heart conditions, the maximum fibrosis was seen in lone AF. It had been demonstrated in the canine model that the fibrosis was accompanied by conduction slowing and heterogeneity occurring in localised areas (2). These provided the substrate for future episodes of AF.
The actual mechanism for atrial fibrosis was unknown; however angiotensin-converting enzyme and angiotensin II appeared to be regulators of transforming growth factor beta1 which was a profibrotic molecule. It could be presumed that atrial fibrosis was reversed with angiotensin converting enzyme-1; this point could have future therapeutic implications. Research in animal models had also indicated that angiotensin II Type I receptor blockers had inhibited fibrosis (2).
Researches had not proved that remodelling of the left atrium was the cause of AF. However the process of development of the persistent AF suggested this possibility. The stimuli that triggered the dynamic process of remodeling could be removed through therapeutic interventions (2). This delayed or halted or even reversed the process of remodeling of the left atrium. If the condition was unattended to, the damage became irreversible. As the underlying pathophysiological processes were still ambiguous, future studies needed to wipe the doubts away and be clear on the underlying mechanism of atrial remodeling (2).
Studies had suggested that the effects of angiotensin-converting enzyme inhibitors, angiotensin II type I receptor blockers and 3 hydroxy-3methyglutaryl- CoA reductase inhibitors reduced the risk for AF where the risk had been high with structural remodeling (40). Prevention of atrial remodeling was to be the point of start of the therapy for AF. Treating hypertension, sleep apnoea, obesity and hyperthyroidism appropriately before AF developed could help. Once the AF had been expressed, efforts were to be on restoration of sinus rhythm as early as possible (2).
External cardioversion
External cardioversion was a treatment for persistent atrial fibrillation (37). Specified energy levels of direct current were passed through electrodes placed in different positions on the chest to send shock wave gradients of 5V/cm. through the fibrillating atria with the aim of conversion of irregular rhythm to sinus rhythm (37). The aim of cardioversion was the restoration of sinus rhythm so that congestive cardiac failure and embolism were prevented (41). Cardioversion was a significant component of treatment that had been employed since the 1960s (42). Alternating and direct current were first used. Then alternating current was abolished and direct current was concentrated upon.
For almost 20 years, direct current cardioversion by the “critically damped monophasic waveform” ruled the roost (42). Inductors and capacitors were part of the application. Soon inductors were found to be too large to use in implantable devices. Next, implantable devices were investigated and capacitor discharges which were truncated were studied (42). Fibrillary activity could be ended by creating a shock-field gradient of 5V/cm. in the arrhythmic myocardium for a short period of a few milliseconds (37).
Closed chest cardioversions were first done in patients who just had heart surgery where it was possible to open the chest rapidly in case of open chest defibrillation becoming necessary (38). Originally done under general anaesthesia, the introduction of intravenous diazepam in 1966 allowed cardioversion to be done in any place where the monitoring of the recovery was possible. The procedure in the 1970s took fifteen minutes. After forty years the idea about cardioversion and its necessity has changed. Presently researchers believe that the procedure may not be as necessary as was thought before. The rate can usually be controlled in many patients by drugs and the necessity for cardioversion has reduced mostly (38).
Younger patients responded to cardioversion faster than patients above 65 years of age. However recurrences had been reported for almost 50% of the younger patients within 3 months (41). Paroxysmal atrial fibrillation patients also had these recurrences. Kuppahally’s study varied from other researches in that it was done in a managed care setting (41).
Transoesophageal echocardiography-guided cardioversion
This was indicated when the period of wait for cardioversion was to be shortened as AF had to be cardioverted as early as possible for better results and when a thrombus had been detected before cardioversion (3). The initial transoesophageal cardiography could reveal a thrombus which increased the patient’s risk for thromoboembolism after the cardioversion. It was necessary to have a normal INR before the cardioversion and this could take many precious weeks. If transoesophageal cardiography did not reveal a thrombus before the cardioversion the thrombus could be located after it. The fatal risk of thromboembolism after cardioversion could be averted by using this facilitated method.
Different waveforms
With improvement in the capacitor technology, waveforms were produced for the cardioversion and implantable devices became smaller and smaller. Later biphasic waveforms were found to be better and more advantageous over the monophasic ones (42). The present trend is to use biphasic defibrillators for external and internal cardioversion. Transthoracic cardioversion using biphasic shocks for the therapy of atrial fibrillation was the focus of study by Walsh et al (42).
At that time there had been no universal guidelines for the procedure and researchers were just switching over from monophasic defibrillators to biphasic ones. Walsh believed that monophasic waveforms produced the same rates of cardioversion as the biphasic with the maximum energy in the treatment protocol. The highest energy used for treatment was used for comparing the efficacy of the biphasic over the monophasic waveforms in his study. At these energies of direct current, damage to the cardiac myocardium was unexpected (42).
Early recurrence of atrial fibrillation could not be treated with biphasic waveforms; the effect would have prevented the atrial remodelling and subsequent fibrosis. Walsh (42) had noted that some researchers had shown that biphasic waveforms were more powerful than the monophasic ones at lower energy impedance even if the monophasic waveforms were of higher energy (42). Monophasic shocks were known to have limitations which gave way to the biphasic cardioversion (43).
Biphasic cardioversion was much better for both internal and external cardioversion. Originally it was for ventricular fibrillation and later began to be used for atrial fibrillation. Biphasic shocks had proved beneficial to cardioversion. New implantable devices with biphasic waveforms had proven to be more successful with a reduced number of shocks in situations where the monophasic had failed and indication for transvenous cardioversion arose. Damage to skin and muscle was also less; myoglobin or troponin I levels were not increased (42).
The monophasic shocks were used frequently in transthoracic cardioversion; they were of the damped sine waveform variety (43). The main limitation of monophasic shocks was the failure to convert. Different researchers had provided differing percentages for the extent of failure of conversion: 10% (44), 20% (45) and 30% (46); these differences could be due to technical variation and features of the patients (43).
Page et al (43) conducted a study to prove the benefit of biphasic shocks over the monophasic. Haemodynamically fit young patients of above 18 years were to undergo elective cardioversion. Patients who had used epicardial defibrillator electrodes or pacemakers were excluded. Participation in another double blind trial was also an exclusion criterion. The use of vasopressors disqualified some patients. Patients who were not able to understand where the defibrillating electrodes were to be placed were also excluded (43).
Based on the INR which was to be equal to or more than two, anticoagulant therapy was provided for 48 hours. For a left atrial thrombus with a negative transoesophageal echocardiogram, IV heparin was given. Anticoagulant was continued for 4 weeks following the procedure. The absence of organized function of the left atrium together with the presence of fibrillary waves and irregularly irregular ventricle was to be the basis of the study (43).
Selection of energy levels
Selection of energy strengths also varied in studies. Mittal had different shock protocols for the monophasic (100J, 200J, 300J and 360 J) while the biphasic waveform shape was rectilinear (43). Shocks were timed to the QRS complex of the ECG (43). In Page’s study, four shocks were given in a protocol from 100J through 150J and 200J and then one other, either 200J or 360J, was given. If all did not work the alternative was to use either the monophasic 360J or the biphasic 200J. At any time that successful conversion of the rhythm occurred or the five-shock sequence was over, the procedure was halted.
The ECG was to show 2 P waves side by side without AF waves seen within 30 secs of the shock (43). Reisinger used 100J initial energy for AF of less than 2 days duration and 150J for those of more than 2 days (47). Stec et al (2011) indicated that rectilinear biphasic cardioversion of low energy was effective in 32-52% of atrial flutter and atrial tachyarrhythmias; an energy of 50 J was sufficient to produce cardioversion in 73% of patients. Patients with pacemakers could also have low energy cardioversions (48). Stec and his colleagues recommended a low energy of 2OJ for the initial energy level of cardioversion in AF (48). Another finding was that 30J in rectilinear biphasic cardioversion was more beneficial than 100J in monophasic cardioversion (48).
If any flutter or fibrillation occurred, the procedure was stopped and interventions made for correction of the arrhythmia. The dermatologist or nurse looked for changes in the skin after 24 hours. There could be erythema of a mild nature and not accompanied by tenderness. A more severe form could be described as moderate erythema and this would be tender to touch. In it most severe form, blistering with necrosis and severe tenderness would be seen (43). This skin change was expected after monophasic shocks. The examiners of the skin change were blinded to the type of shock used, whether mono or biphasic in Page’s study (43).
Manufacturers had the tendency to recommend different energy selections as the best for initial shocks. AF of short duration could be successful with shocks of lower energy. Studies however indicated 80% success rate with 150J (42). Other manufacturers recommended the efficacy of 360J. However studies spoke for 200J as sufficient for cardioversion; very few patients required a higher energy. A multicenter had used single energy shocks of 200J without any escalation; first shock success was higher. There had been a comparison with escalating energy (42).
Are biphasic shocks superior to monophasic?
The efficacy of biphasic over the monophasic waveforms had been attributed to the calcium movement into and out of the myocytes through the ion channels by some researchers. However the electrophysiology was still a mystery in the fibrillation as well as the biphasic waveforms. Several mechanisms had been proposed. One says that the electrode polarization in the first phase reduced the impedance in the second phase and thereby improved the efficacy of the biphasic waveform (49).
This explanation was not valid as some biphasic shocks had larger defibrillation threshold difference even if their second phase had lower impedance. Another mechanism proposed is the huge difference in peak to peak voltage between the two phases. This also did not hold good as two phases could be at an interval of even 6 msecs, hence the change of peak voltage was discarded and the biphasic superiority did not suffer (49).
The view that biphasic shocks produced lesser damage to the myocardium and better stimulation of the refractory myocardium was true. That sodium channel activity was restored during the first phase of the biphasic shocks and the refractory period was extended have been disproved by researchers (49).
This was because the channel restoration theory could not explain the reason for the superiority of the biphasic shocks over the monophasic ones. Some researchers believed it to be due to the virtual electrode effect, an area of the heart itself behaving like an electrode. Others thought it was due to the charge burping theory (49). The charge burping theory stated that the areas of virtual electrodes in the myocardium produced in the first phase were eliminated by the second phase (49). Biphasic shocks were less potent in the extension of the refractory period and so this mechanism did not explain the superiority of biphasic shocks.
Biphasic superiority could be explained by the fact that they produced greater post shock repolarization if delivered in the sinus rhythm (49). Documentation of virtual electrodes (hyperpolarized areas found across a depolarized region) had suggested that shocks could fail; the same mechanism had been suggested for efficacy of biphasic shocks. The adjacent positions of the depolarized and hyperpolarized tissue could lead to defibrillation shock failure. A strong first phase followed by a weak second phase seen in biphasic shocks was just right for defibrillation (49).
The calcium transients following the biphasic defibrillation shocks were studied by Hwang et al (50). The first activation was found in the area of low intracellular calcium and noted 50-60 ms post-shock (49). The calcium transient was found more heterogenous after monophasic shock (50). If thapsigargin or ryanodine was administered, the better performance of the biphasic shock could be answered by the calcium transient. Hwang’s explanation was acceptable except for a few limitations. One was that only the epicardium had been studied; the deeper tissue could not be studied by optical mapping (49). However the focal activation pattern from the calcium sinkholes could be arising from the deeper tissue.
If the theory that calcium transients accounted for the success of the biphasic shocks was valid, the failed shocks of both monophasic and biphasic have depolarization and calcium transient maps which are similar and these have not been explained (49). This was also a limitation of the study. One point which contributed valuable information was that the peak depolarization occurred earlier for the biphasic shocks just like the peak calcium. The possibility that the calcium transients could be due to another process like the extension of the refractory period was another contention (50).
Position of electrodes
Cardioversion was better in the patients with antero-posterior electrodes. It had also been suggested that even the antero-posterior positions could have variations. Theories had been suggested by many researchers for the various positions of the electrodes (42). Some researchers said that when both atria were involved in the fibrillation, the position of electrodes in the right anterior and left posterior position was best.
If the left atrium was most affected, the position of left anterior and right posterior was believed to be better (42). The anterior-anterior position did not allow most of the current to reach the atria; only 4% did so while the rest just travelled around the thoracic cage. Different techniques had been described by different researchers. Some placed the electrode in alternate position compressing the electrodes. Others used two defibrillators to provide simultaneous shocks (42). The prior infusion of Ibutilide had raised the success rate of cardioversion to 100% (42). The risk of arrhythmia due to the infusion was a disadvantage.
Though current guidelines accepted both positions of the electrodes, the electrodes in the anterior-posterior position were believed to be better than the anterior -lateral position for providing a uniform shock gradient in the atria (37). This was explained by the position of the atria which lay one behind the other and it enabled the flow of maximum current through the atria (51). Also the transthoracic impedance was lower for this position of electrodes. There had been arguments about the success of cardioversion in the two positions; some were for the position while others were not (37).
Kirchhof studied the advantages of both positions (37). The participants who had persistent atrial fibrillation underwent elective cardioversion using the step-up protocol of energies from 50J to 360J. The electrode positions were random. The position of the electrode was changed if the initial position did not support a conversion at 360J. More patients had successful cardioversion in the anterior-posterior electrode position at 96%. Twelve patients showed a response in a cross-over of position from the anterior-lateral to anterior-posterior (37). Cross-over in the other direction was successful only in two patients. Following the cross-over, 102 out of 108 patients had successful cardioversion.
Cardioversion was deemed successful if the fibrillary activity was terminated and sinus rhythm prevailed. Seventy patients of the 108 had comorbidities of the heart. AF persisted for 5 months. Anti-arrhythmic drugs were being given to 80 patients (74%) (37). Amiodarone was being administered in 20 patients. 52 patients had already been cardioverted once before. Kirchhof indicated that the success of prolonged sinus rhythm following cardioversion depended on two factors. The atrial modeling was one factor which without doubt inhibited success. Structural changes also could interfere with the success of cardioversion. The presence of focal triggers was another possibility of lesser success (37).
The electrodes used in Page’s study were identical and placed anterior-posterior. The posterior electrode was placed at the midscapular level to “the right of the spine” (37). The position of the anterior electrode was to the left side of the sternum at the 4th intercostal space. Data was obtained using a 12-lead ECG. The defibrillator recorder was also functioning. A standard unit was used for the monophasic device. The biphasic device provided an impedance-compensated waveform (37).
An efficacious position had been studied by Kirchhof in 2005: the midline antero-posterior position (52). The influence of transthoracic impedance on the success of cardioversion using a modern defibrillator and using the midline AP position led to some interesting results. Walsh et al (42) compared the success of cardioversion in monophasic waveforms which were not compensated with transthoracic impedance and biphasic waveforms which were minimally compensated using hand-held paddles. (42).
Walsh in another study found that cardioversion success was associated with the antero-posterior position which was also associated with less transthoracic impedance in most patients (42). However the outcome of cardioversion using the AA position with DC current has been improved using modern defibrillators which had the facility of impedance compensation (42). The drawbacks of electrode position do not interfere with the results when using a modern defibrillator where the influence of transthoracic impedance is minimal.
Transthoracic impedance
Though many scientists had claimed the advantages of the various electrode positions in different waveforms, nobody bothered to measure the transthoracic impedance till Walsh and his colleagues in 2005 (42). The innovative biphasic defibrillators however promoted cardioversion at different thoracic impedances. The patients with lesser transthoracic impedance (smaller patients) were compensated with lesser voltage and current while the ones with larger transthoracic impedance (heavier and bigger patients) were treated with higher voltage and current (42).
The analysis of a study
Data analysis in Page’s study was reviewed every month by the monitoring committee, blinded to the procedure used (43). 105 patients had been targeted so that after dropouts at least 94 remained for each arm..The assessment was made after six months at the termination when the monitoring committee was unblended (43). The rhythm changes in the ECGs and the measurement of the ST segments were performed by cardiac electrophysiologists. The Fisher exact test was used for analysing the binary counts. Adjusted relative risks and the confidence intervals were calculated with the Fisherman-Freeman-Halton test (StaXact version 4.0.1).
The results were elicited after adjustment for age, gender, weight, class of the heart failure, ejection fraction and left atrial size. It was found that 14% of patients were converted with monophasic shocks while conversion occurred for 6% of the biphasic group (43). Less skin injury was seen after the biphasic. Predictors of the first shock success were the weight of the patients, period of the present AF and transthoracic impedance (43). For the two kinds of shocks, the increase in both weight and transthoracic impedance inhibited the cardioversion at the first shock (43). The monophasic population which did not convert within the four shocks had higher transthoracic impedance. Following conversion, more of the biphasic patients (97%) were in sinus rhythm than the monophasic group (91%).
Obviously the biphasic shocks were better than the other. Success was greater with each new shock energy in both types in the step-up manner. One significant advantage for the biphasic shock was that lesser skin injury was produced and lesser shocks were required for cardioversion (43). The best predictor of initial shock success was the biphasic one. Lower weight, a lesser duration of AF and reduced transthoracic impedance were also independent predictors for both the defibrillators.
If considered between the first four shocks, the lesser transthoracic impedance would be the only independent predictor; this having an influence only in the monophasic type (43). Reisinger et al after their study concluded that impedance-compensated biphasic shocks ruled out the influence of transthoracic impedance but the success rate was still dependent on weight and sex (47).
The biphasic superiority was demonstrated by Ricard et al (44) and Mittal et al (45). Ricard’s study had been small producing limitations in the results. There were only two classes of AF in Ricard’s study; the period of AF considered as short had AF for less than 7 days and the chronic had more than 7 days of AF (44). Mittal’s classes of AF were similar to Page’s but the really long duration AF patients (more than a year) were not a separate group (45).
Another difference in Mittal’s study was that he had 19% of short AF compared to 13% in Page’s group and 13 % of longer duration AF compared to 25% in Page’s (45). This probably accounted for the difference in the efficiency of the four shocks: 94% in Mittal’s and 91% in Page’s. Mittal’s (45) and Ricard’s (44) studies had the possibility of investigator bias as they had no separate event committee. Mittal’s (45) results of the study which used the rectilinear biphasic shock and Page’s results were similar, however the possibility of altered results with other waveforms of biphasic shock still stood as a limitation of Page’s study (43).
Page’s study concluded that the apt first choice of biphasic waveform to be used would be having an energy of 200J and it would be just right for patients having AF for more than a year (43). For AF of less than 48 hours duration, 80 % conversion was possible with 100J. If 200J did not produce a conversion, changing the electrode could clinch the cardioversion. Antiarrhythmics could be given followed by the shock. Double shocks or catheter cardioversion could be tried (43).
Technicalities of the cardioversion procedure for atrial fibrillation by a manufacturer
Minor technical details may ensure that successful cardioversion occurs. The following recommendations were made by the Zoll Medical Corporation with the assistance of Mark Niebauer (51) who had himself conducted a study which compared the rectilinear biphasic waveform with damped sine monophasic waveform. Cardioversion was best with electrodes in the antero-posterior position. The anterior pad was positioned adjacent to and close to the right lateral portion of the sternum (53). The midline of the electrode was to be in the fourth intercostal space. The posterior electrode was centred at the level of T7 vertebra.
If a solid gel electrode was used, the active area would be the tin on the electrode. If a wet gel electrode was used, the foam on the gel was the active area. Wet gel electrodes were preferred to produce the most effective coupling of skin and gel. The skin preparation was important: a dry skin surface with no hair or lotions would be suitable. Pads needed to be applied carefully: The posterior one was first rolled onto the skin to produce good adhesion without air pockets.
The ECG was to be timed on the R wave (53). One minute rest time was given between shocks. Betablockers could produce shock bradycardia. Transcutaneous pacing was advantageous for the patient who had bradycardia following the shock until his rhythm became normal. The patient in the antero-posterior position had the advantage of the immediate start of his pacing as the sternal pad was easily moved to the left side (53).
Less bulky patients with short duration AF usually converted with the first shock. In order to protect the myocardium as much as possible and ensure high efficacy at the first shock itself, 75J was considered ideal (52). A higher first dose of 100J to 120J was better for patients with persistent atrial fibrillation as a 90% success would be seen. If the patient was not easy to convert, a first dose of 200J was useful (52). Pressure on the pads helped by reducing the distance for the optimal flow of current. The pads could be moved to other positions if the first shock was unsuccessful. The sternal pad could even be moved to the other side of the sternum for a few patients (51)
Factors affecting success of cardioversion
The success of cardioversion could last for a long time. There were certain factors on which the success depended. Reducing the risk of stroke was the main target of treatment. The predictors of stroke were many. Age above 75 years was one; less success was associated with older patients. Cardiac illness like mitral stenosis which was prone to thromboembolism was a predictor of stroke. Hypertension whether treated or not predicted stroke. A previous episode of transient ischemic attack or a stroke could influence the success of cardioversion by repetition at any time. Congestive heart failure was a condition which had earlier remodelling changes which could decide the success of a cardioversion.
Left ventricular dysfunction which impinged on the ejection fraction could also inhibit success (54). The risk of stroke following cardioversion was between 6-7% and this was really high; the administration of anticoagulant greatly reduced this risk to below 1% (55). Warfarin was administered to patients above 75 years if they already had any of the risk factors. For patients between 65 and 75 without risk factors, Warfarin or aspirin was used.
For patients below 65 with no risk factors, aspirin was used but its action had not been studied yet (54). The INR result was kept between 2 and 3 while Warfarin was administered; the dose varied accordingly. Anticoagulation with Warfarin was started 3 weeks before the cardioversion after ensuring sinus rhythm for 4 weeks for AF with short duration illness. The anticoagulation is thus a factor for successful cardioversion (53).
The age of the patient was one factor which had a role to play in the success of cardioversion according to a manufacturer, Zoll Medical Corporation (53). Digoxin, beta blockers and calcium channel blockers were used to influence the heart functions. The AV nodal conduction could be blocked. Simultaneously the ventricular rate could be decreased and the ventricular function thereby improved (53). Older patients above 75 could be given digoxin which was effective in increasing the contractility of the ventricle and its function simultaneously. As digoxin was not as effective as beta blockers or calcium channel blockers, atenolol could be combined with digoxin to produce a beneficial effect (53). The beta blockers and the calcium channel blockers had the disadvantage of exacerbating the congestive heart failure.
The younger age group below 75 had a better chance of maintenance of their sinus rhythm (56). Kuppahally’s study of 2009 indicated results differently: age had no influence over the success of cardioversion (41). With increasing energy levels from 50J to 360 J, the success for both younger and older patients, the cut off age being 65 years, was similar for the initial shock of 50J of the cardioversion. After one year, the younger patients already had new episodes of AF.
The relapse rate for the ones younger than 65 was 35% while it was lesser at 22% for the older ones (41). This was interpreted as the recurrence of AF for younger patients being twice that for older ones. The Framingham study found a relationship between the two when the alcohol consumption was more than 3 drinks per day (41). There was no relationship when the consumption was less. Rate control strategy was preferred for the alcoholic patients (41).
Gender variation had been reported by Kuppahally (41). Of the females, 72.5% had successful electrocardioversion while 62 % only of the males had it. However when comorbidity was adjusted for, the difference did not arise.
Comorbid conditions however did not really affect the success of cardioversion. Paroxysmal atrial fibrillation patients with alcoholism had more episodes of recurrence.
Treatment with beta blockers had been found to be an independent factor for successful cardioversion by early researchers like Tieleman et al (56). Frick et al also concluded that administration of beta blockers was an independent factor. Blich and Edoute have confirmed this suggestion in their study in 2006 (56).
Obstructive sleep apnoea was found to be associated with cardiac illnesses and so this also had been studied as a factor which affected the success of cardioversion (62). However some researchers believed that this problem was found in obese patients and obesity could be the predisposing factor.
Weight or obesity was a predictor of success of cardioversion (56). Greater body weight and BMI more than 30 was related to greater electrical impedance where the direct current shock to the atria was impaired causing unsuccessful cardioversion (56). The patients of normal weight or less and who had a BMI less than 30 could be expected to have cardioversion success. A higher energy was provided for the obese (56).
Hypertension was previously not considered a factor for successful cardioversion: it was a risk factor later included (56). However the presence of hypertension could be inducing alterations in the myocytes: a greater level of sympathetic activity could be causing many new reentrant wavelets within the atria. Changes could be involving the sinus node too. This probably explained the reason for the sinus node not being able to resume its normal function of pacemaker after the electrical cardioversion when the several new reentrant wavelets were depolarized (56).
Electrophysiological properties of the atria were altered during AF making them increasingly prone to problems (56). The atria underwent remodeling as a result of the fibrillary waves. Cardioversion could not be successful following the remodeling. The excessive increase of intracellular calcium could be involved in the AF-induced atrial remodeling. Researchers have suggested that administration of calcium channel blockers which reduced the excess intracellular calcium could prevent a relapse in the cardioversion. The beta adrenergic blockers were the calcium channel blockers which lowered the intracellular calcium and could therefore be expected to lower the relapses (56). Other researchers had included the treatment with calcium channel blockers as an independent factor but Blich and Edoute (56) could not justify this.
Recurrence needed to be prevented if success was to prevail. Drug therapy could be a factor which ensured success by preventing the recurrence but this was controversial (54). Studies had suggested that the maintenance of sinus rhythm had been possible with drugs but mortality could still occur. The role of beta blockers in prevention of recurrence had to be studied further. Amiodarone seemed to be effective and safe but recurrence was high at the end of a year. The new drug dronedarone was now more accepted as it did not possess the adverse effects of amiodarone (18). Researchers had suggested that new AF cases could be successfully cardioverted while a first relapse could be dealt with by cardioversion and the drug amiodarone (54).
Paroxysmal AF was a recurrent type of AF and drug therapy could help reduce the recurrence: sotalol, fleicanide and propafenone increased the interval between the episodes but did not abolish them. Kuppahally also recommended the use of antiarrhythmics for AF before the cardioversion (41). The adverse side effects of the drugs needed to be weighed against the problems arising from delay of cardioversion before making a decision to use drugs for maintaining sinus rhythm. Relapses were not reduced by the use of these drugs in Kuppahally’s study (41).
Left atrial size did have an influence in cardioversion; mild or moderate enlargement was not a contraindication for cardioversion (56). However Kuppahally had reported just the opposite (41). The observation was that short or long-term success was not influenced by left atrial size. The left ventricular functions also had no relationship with the success of the initial external cardioversion. The patients who had a history of alcoholism were influenced by left atrial size: those who had more than 4.2 cm. In this group too, the younger patients had more recurrences.
Congestive heart failure was also a predictor of successful cardioversion but if mild or moderate, it was not a contraindication (56). The rhythm control was favoured for the patients with congestive heart failure by some researchers. It could be also the manner of treatment for AF patients who have disabling features (41).
Duration of AF if less than a year was not considered a contraindication to cardioversion (56). Kuppahally et al (41) indicated an inverse relationship of duration of AF with success of cardioversion as seen in the following Table 3. Gallagher’s (57) results are given in Table 4 on page 66
Table 3 Relationship between duration of AF and success rate of cardioversion (41)
A similar comparison had been made by Gallagher and his colleagues in 2001(Table 4 below). Their study lasted a longer period and they were able to follow-up their patients for 5 years (57). In both studies the best success rate had been recorded for the period immediately after the cardioversion. It iwas interesting to note that 50% success was still present 5 years after cardioversion in Gallagher’s study.
Table 4 Relationship between duration of AF and success rate of cardioversion (57)
Kuppahally indicated that the long term recurrences after the cardioversion were not influenced by duration of AF (41). Borriani (55) predicted success for a duration of AF of more than a year. As researchers had come up with these different conclusions, it could be assumed that AF of any duration could be cardioverted. Seventy-two percent of cardioverted AF patients remained in normal sinus rhythm till just before 1 year; this was reduced to 53% at the one year follow-up. This was different from the AFFIRM study which reported 42%. It was interesting to remember that Taylor (54) had a different definition to success. He suggested that the maintenance of sinus rhythm could not be the main target of success. Instead the ventricular function should be maximized and the risk of stroke reduced (54).
Persistent AF patients underwent many alterations in the pathology, electrophysiology and anatomy of the atria causing their refractoriness (41). Higher incidence of failure was the result. Cardioversion was to be performed as early as possible. These cases of refractory AF needed innovative techniques like the radiofrequency catheter ablation or pulmonary vein ablation. Pulmonary vein ablation seemed to improve quality of life in persistent AF patients by the decrease of recurrences (41). Other researchers had recommended the Cox-Maze procedure for younger patients (41).
If sinus rhythm had been maintained for 6 months after the first cardioversion, relapses could be indications for repeated cardioversions.
Implantable cardioverter defibrillators
The technological innovation of the first generation implantable cardioverter defibrillators had marked a great step forward in the treatment of atrial fibrillation and other arrhythmias (58). Atrial defibrillators diagnosed arrhythmias of the atria and provided prevention and termination therapies (59). Atrial therapies could be restricted to periods of the day more acceptable to the patients or limited to lesser number of therapies. Atrial arrhythmias were not life threatening and so the therapy needed to be done with a view to improvement of quality of life and symptom reduction (59).
The efficacy and accuracy of these machines had increased over time. Dual chamber devices allowed the dual atria pacing and provided additional information. The first device was not accepted by patients due to the painful procedure (60). That device did not have diagnostic abilities. They required a thoracotomy for placing the epicardial patch and hospitalisation was required for one week.
Currently many devices which are automatic or commanded have appeared in the market for the atrial fibrillation therapy. The devices are really meant for ventricular fibrillation and can be simultaneously used for atrial fibrillation. Though many devices have appeared clinical practice has not fully enjoyed them (60). Combined atrial and ventricular devices have been recommended for AF patients with congestive cardiac failure.
In the course of time, ICDs would be easier to use and better than the previous ones. The biventricular pacing devices (CRT-D) are the ones progressing really fast (60). Biphasic waveforms will continue to be used for some more time till another waveform is found to be better. The battery and the capacitors may show alterations and improvement. At some point of time if medical therapy is sufficient to improve the condition of a patient with reduced ejection, these devices may disappear (60).
The annual mortality from congestive heart failure was placed at 20-40% in the 1980s when the first generation devices appeared and could be used for 3 years. Patients did not expect to live longer. The annual mortality has reduced drastically to 7% in patients who are now having “angiotensin-converting enzyme inhibitors, aldosterone antagonists or beta blockers” (60). Device longevity has thus become a significant requirement. For the institution of devices, the diagnosis has to be correct initially. Prevention of ventricular arrhythmias is not a well-developed procedure. Overdrive pacing is one therapy for ventricular arrhythmias. A drug pump may be used to release antiarrhythmics locally. The cardiac afferent nerves could also be stimulated to trigger a series of nerve-mediated changes.
Early detection of heart failure worsening could be possible by combining intravascular haemodynamic monitors with the ICDs. This same method forms the trigger for pacing or drug pumps (60). Myocardial ischaemia may be detected and therapy started by using the haemodynamic monitor with the device. The modern devices provide plenty of information regarding the rate and morphology of ECG signals before starting therapy, during the therapy and following it (60). Improvements in the lead design, waveforms and the minute sizes enable the defibrillator to be used as an implantable device just like the pacemaker. The longevity is now 6 years. Efforts may be made to discover an ICD which can last for many more years.
Mortality due to sudden cardiac death may be primarily or secondarily prevented by the implantable devices (58). Mortality is thus reduced but at a cost. One limitation of defibrillators is that patients can be mentally and physically affected, the consequence being a poor quality of life. The psychocognitive responses could be maladaptive ones making the patients anxious and depressed (58). Pain of shock may disturb the patient. Occasionally some shocks produce arrhythmias. The subsequent risk of death was reported in two study trials: Multicenter Automatic Defibrillator Implantation Trial II (MADIT II) and the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) (58).
The cause is still evasive. The ill-effects and high costs of shocks project the idea that they must be reduced. Anti-tachycardia pacing (ATP) is one strategy for reducing shocks. Prolonging the time for detection is another. Inappropriate shocks must be avoided. The supraventricular tachycardia discrimination needs to be better (58). Combining the antitachycardia pacing with shocks could lead to failure; the tachycardia may be increased and this causes undue delay in the therapy. Sudden death or syncope after the therapy must be assessed to be the failure of the combined therapy.
Optimal programming for device therapy in atrial conditions may need to follow certain guidelines (59). Pacing therapies may be freely used; the main goal being freedom from symptoms and better quality of life. Therapy with shocks must be avoided if possible. If possible, conversion must occur after the first one. Pain is more associated with increased number of shocks rather than a single one of high intensity (59). Some patients like to perform the pacing therapy themselves as soon as they detect arrhythmias.
Patients must be guided and allowed a test shock to inform them about the self- administration of the therapy (60). The patient must be familiar and comfortable with using the activator. Optimal programming helps to reduce the risk of shocks whether considered appropriate or not. The comorbidities expected like heart failure and atrial fibrillation could be monitored and prevented or treated immediately. Battery longevity must be ensured. Malfunction of the heart must be noted in time for intervention. Clinical symptoms should not be waited for (60).
Society does have a long way to go to find a suitable solution to preventing AF or preventing a progression to atrial remodeling after cardioversion or keeping the heart in sinus rhythm. The anticoagulant, drugs for rate and rhythm controls with high efficacy and more efficient implantable cardioverter defibrillators, which monitor and provide the therapeutic shocks, are necessary to lengthen a life of high quality in the aging population.
The preventive management immediately after the cardioversion prevents the atrial remodelling which perpetuates further AF. Complications of stroke or cardiac failure will also be prevented with the apt management. Maybe there will come a day when even cardioversion becomes unnecessary; there is a school of thought in this direction already. Future research may be more reliable and comparable if based on the NCC-CC Guidelines where a classification has been proposed and which cater to an international population.
The writer’s impressions
Atrial fibrillation is a fairly fatal illness where the atria perform in an irregular manner with subsequent reduction of mechanical function. Being the commonest arrhythmia in clinical practice, it is known to have a fatal ending leading to a rise in mortality. Sudden death, congestive heart failure and stroke are the usual consequences of atrial fibrillation and all are fatal or associated with a poor quality of life.
The huge financial burden on the nation of this major global health issue must attain a protocol for preventive management of atrial fibrillation and successful cardioversion for patients with AF, reducing the morbidity and mortality of AF, preventing the complications and improving the quality of life of patients with AF. A better understanding of the underlying mechanisms and the damaging alterations in the heart muscle following AF through further research is expected to spearhead a solution in the near future. The problem is growing into gigantic proportions in direct relationship to the size of the aging population which has available options for treating effectively conditions like atherosclerosis and obesity (2).
Research has identified specific areas which are hypothesized to be drivers: the left atrial appendage and the area outside the left pulmonary veins (1). Excitable gaps within the atrial fibrillation provide the possibility of capture of the atrium while fibrillation is proceeding (1). It may be presumed that atrial fibrosis may be reversed with angiotensin converting enzyme-1; this point may have future therapeutic implications. Research in animal models has also indicated that angiotensin II Type I receptor blockers have inhibited fibrosis (2). The therapy is started early to prevent atrial remodelling.
Electrophysiological classification had no benefit for clinicians for management of AF. The clinical classification provided in the NCC-CC Guidelines will hopefully be abided by uniformly by all clinicians for the sake of management of AF. Future research should be based on the entities as described in the Guidelines. Inferences should in future be comparable and provide deep insights into the mechanism of AF presentation and its management, both preventive and otherwise.
Prognostic therapies are to be focussed upon rather than remedial ones. Therapy has to be an active process especially in the new-onset AF. A window period has been recognized when the institution of therapy provides best results. The aggressive therapy will not work when atrial fibrosis is extensive. AF as a new disease must be treated to re-initiate sinus rhythm and maintain it by rhythm control. This will be essential if the patient is expected to live long for more than 3 years. The possibility of reverse modeling is high. Some patients may require antiarrhythmic therapy after cardioversion. Catheter ablation seems to be a better proposition than antiarrhythmic drugs if study results are to be believed; however more research is necessary to prove this.
Dronedarone has been approved by the Food and Drug Administration for use in AF. The dosage of 400 mg. was used in EURIDIS and ADONIS trials. Both these studies showed a larger interval to the first recurrence of AF and a reduction in the ventricular response in the recurrences. The ATHENA concluded that cardiovascular morbidity and mortality could be decreased by the use of dronedarone. It could positively reduce strokes in aging patients and in the treatment of older patients who were ailing with non-permanent AF.
It forms “the first line therapy for atrial fibrillation or flutter in patients with structural disease who have an EF of > 35 % with no recent decompensated heart failure” (Wolbrette et al, 2010). Though not much better than amiodarone it positively reduces hospitalization for cardiovascular illnesses and does not have the side effects of amiodarone. Lower health costs may be expected from the use of dronedarone.
The recent approaches to cardioversion include the use of pacemakers or defibrillators. Catheter ablation techniques are another novel approach. Elderly patients who do not have contraindications may be selected for linear ablation and pulmonary vein ablation. Patients who cannot have drug therapy for atrial fibrillation for rate or rhythm control may have the ablation methods. Focal AF may be managed with radiofrequency ablation (4). Kirchhof thinks that the future studies should attempt at improving the method of cardioversion for the socio-economic benefit of humankind (37).
Page’s study concluded that the apt first choice of biphasic waveform to be used in defibrillators would be having energy of 200J and it would be just right for patients having AF for more than a year (43). For AF of less than 48 hours duration, 80 % conversion is possible with 100J. If 200J does not produce a conversion, changing the electrode position could clinch the cardioversion.
The present trend is to use biphasic defibrillators for external and internal cardioversion. The risk of stroke following cardioversion is between 6-7% and this is really high; the administration of anticoagulant greatly reduces this risk to below 1% (55) Warfarin is used as the anticoagulant of choice except for patients with seizures, gait problems and syncope before cardioversion (25). The relapse rate for the ones younger than 65 was 35% while it was lesser at 22% for the older ones (41).
Researchers have suggested that administration of calcium channel blockers which reduce the excess intracellular calcium may prevent a relapse in the cardioversion. The best success rate has been recorded for the period immediately after the cardioversion.
Atrial defibrillators diagnose arrhythmias of the atria and provide prevention and termination therapies (59). The biventricular pacing devices (CRT-D) are the ones progressing really fast (60). Combining implantable defibrillators with intravascular haemodynamic monitors can help prevent sudden cardiac death and impending ischaemic heart disease. The advantages of optimal programming are “lowering the risk of appropriate and inappropriate shocks, reduction in the level of comorbidities like congestive heart failure and atrial fibrillation, increase in the battery longevity and detection of malfunction before it is clinically diagnosed” (60).
References
- Josephson ME. Clinical Cardiac Electrophysiology: techniques and interpretations. Lippincott, Williams and Wilkins. 2008.
- Anter E and Callans DJ. Pharmacological and electrical conversion of atrial fibrillation to sinus rhythm: Is it worth it? Circulation 2009; 120:1436: 1443.
- National Collaborating Centre for Chronic Conditions. Atrial fibrillation: national clinical guideline for management in primary and secondary care. London Royal College of Physicians.
- Papp JP, Straub M and Ziegler D. Atrial fibrillation: New therapeutic concept. IOS Press, Netherlands.2003.
- ACC/AHA/ ESC guidelines for the management of patients with atrial fibrillation European Heart Journal 2001; 22: 1852-1923. Web.
- Conover, MB. Understanding electrocardiography. Elsevier Health Sciences. 2002.
- National Heart, Lung and Blood Institute. Web.
- Erlich JR, Couto P, Yeh YH,Qi X and Nattel S. Cellular electrophysiology and the substrate for atrial fibrillation in Contemporary Cardiology: Atrial fibrillation from bench to side (Eds.) Natale A and Jalife J. Humana press, Springer Science and Business Media.LLC 2008.
- Natale A and Jalife J. Contemporary Cardiology: Atrial fibrillation from bench to side. 2008 Humana press, Springer Science and Business Media.LLC.
- Schweizer PA,Becker R, Katus HA, Thomas D. Dronedarone : Current evidence for its safety and efficacy in the management of atrial fibrillation.Drug Design, Development and Therapy 2011; 5:27-39.
- Sanders P, Berenfeld O, Hocini M et al. Spectral analysis identifies sites of high frequency activity maintaining atrial fibrillation in humans Circulation 2005; 112: 789-797.
- American Heart Association 2002. Heart and stroke statistical update. Dallas TX. American Heart Association.
- Friberg J, Scharling H, Gadsboll N, Jensen GB. Sex-specific increase in the prevalence of atrial fibrillation (Copenhagen City Heart Study). ) American Journal of Cardiology 2003;92(12):1419-1423.
- Healey JS and Connoly SJ. Atrial fibrillation: hypertension as a causative agent, risk factor for complications and potential therapeutic agent. ) American Journal of Cardiology 2003; 91(10A):9G-14G.
- Aviles RJ, Martin DO, Apperso- Hansen C, Houghtaling PL, Rautaharju P, Kronmal RA et al. Inflammation as a risk factor for atrial fibrillation.Circulation 2003;108(24): 3006-3010.
- Eaker ED, Sullivan LM, Kelly-Hayes M, D’Agostino RB Sr, Benjamin EJ. Anger and hostility predict the development of atrial fibrillation in men in the Framingham Offspring study. Circulation 2004; 109(10):1267-1271.
- Darbar D, Herron KJ, Ballew JD, Jahangir A, Gersh BJ, Shen WK et al. Familial atrial fibrillation is a genetically heterogenous disorder. Journal of American College of Cardiology 2003; 41(12):2185-2192.
- Christiansen CB, Torp-Pedersen C and Kober L. Impact of Dronedarone in atrial fibrillation and flutter on stroke reduction. Clinical Interventions in Aging 2010;5:63-69 Published by Christiansen et al, Dove Medical Press.
- Camm AJ. Quality of Life in patients with Atrial Fibrillation. Rev Esp Cardiology 2010; 63(12): 1393-1395.
- Saksena S. Electrophysiological disorders of the Heart.Elsevier Health Sciences:2005.
- Padanilam, B.J. and Prystowsky, E.N. (2008) Epidemiology of atrial fibrillation: The rising prevalence in Contemporary Cardiology: Atrial fibrillation, from bench to bedside. (Eds.) Andrea Natale and José Jalife, Humana Press, Totowa, NJ. Springer publication.
- Topol EJ and Califf RM. Textbook of Cardiology. Lippincott Williams & Wilkins, 2007; Philadelphia PA. p. 579.
- US Census Bureau. Projections of the total residential population by 5 year age groups and sex with special age categories: middle series 2050-2070. Washington DC, Population Projections Program. Population Division, US Census Bureau 2000.
- Go AS. The epidemiology of atrial fibrillation in elderly persons: the tip of the iceberg. American Journal of Geriatric Cardiology 2005; 14:56-61.
- Singh, B.N. and Sarma,J.S.N. Mechanisms of anti-arrythmic actions in the maintenance of sinus rhythm in patients with atrial fibrillation: Clinical and experimental co-relations in Atrial fibrillation: New therapeutic concept (Eds.) JG Papp , M Straub and D Ziegler. IOS Press, Netherlands.2003.
- Corley SD, Epstein AE, DiMarco JP, Domanski MJ, Geller N, Greene HL et al. Relationships between sinus rhythm, treatment and survival in the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) Study.Circulation 2004;109:1509-1513.
- Wolbrette D, Gonzalez M, Samii S, Banchs J, Penny-Peterson E and Naccarelli G. Dronedarone for the treatment of atrial fibrillation and flutter; approval and efficacy. Vascular Health and Risk Management Journal 2010;6:517-523 Published by Wolbrette et al, Dove Medical Press.
- Carlsson J, Miketic S, Windeler J, Cuneo A, Haun S and Micus S et al. Randomised trial of rate control versus rhythm control in persistent atrial fibrillation. : the Strategies of Treatment of Atrial Fibrillation Journal of American College of Cardiology 2003; 41:1690-1696.
- Roy D, Talajic M, Nattel S, Wyse DJ, Dorian P and Lee KL et al. Atrial fibrillation and Congestive Heart Failure Investigators. Rhythm control versus rate control for atrial fibrillation and heart failure. New England Journal of Medicine 2008;19(358):2667-2677.
- Miyasaka Y, Barnes ME, Bailey KR, Cha SS, Gersh BJ, Seward JB, Tsang TS. Mortality trends in patients diagnosed first with atrial fibrillation: a 21 year community =based study. Journal of American College of Cardiology 2007; 6(49): 986-992.
- Swedberg K, Olsson LG, Charlesworth A, Cleland J, Hanrath P, Komajda M. et al. Prognostic relevance of atrial fibrillation in patients with chronic heart failure on long term treatment with beta blockers: results from COMET. European Heart Journal 2005; 26:1303-1308.
- Stabile G, Bertaglia E,Senatore G, De Simone A, Zoppo F and Donnici G et al. Catheter ablation treatment in patients with drug refractory atrial fibrillation: a prospective multi-centre randomized controlled study European Heart Journal 2006; 27:216-221.
- Wazni OM, Marrouche NF, Martin DO, Verma A, Bhargava M and Saliba W et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line of treatment of symptomatic atrial fibrillation: a randomized trial.JAMA 2005;293:2634-2640.
- Noheria A, Kumar A, Wylie JV Jr., and Josephson ME. Catheter ablation Vs antiarrhythmic drug therapy for atrial fibrillation: a systematic review. Archives of Internal medicine, 2008;168: 581-586.
- Morillo C, Natale A. RAAFT: Radiofrequency Ablation versus Antiarrhythmic Drugs for Atrial Fibrillation Treatment. Hamilton, Ontario, Canada: Population Health Research Institute;2006.
- Bertaglia E, Stabile G, Senatore G, Collela A, Del Greco M and Goessinger H. A clinical and health-economic evaluation of pulmonary vein encircling ablation compared with antiarrhythmic drug treatment in patients with persistent atrial fibrillation(Catheter Ablation for the Cure of Atrial Fibrillation-2 study). Europace 2007; 9:182-185.
- Kirchhof P, Eckardt L, Loh P, Weber K, Fischer R-J, Seidl KH et al. Anterior-posterior versus anterior-lateral electrode positions for external cardiversion for atrial fibrillation: randomised trial. Lancet 2002; 360:1275-1279.
- Silverman ME. History and personal observations of electrical cardioversion of atrial fibrillation. American Journal of Cardiology 2004;94:751-752.
- Issa ZF, Miller JM and Zipes DP. Clinical Arrhythmia and Electrophysiology: a companion to Braunwald’s. Elsevier Health Sciences 2008.
- Healey S, Baranchuk A and Crystal E. Prevention of atrial fibrillation with angiotensin converting enzyme inhibitors and angiotensin receptor blockers: a meta-analysis Journal of American College of Cardiology 2005;45:1832-1839.
- Kuppahally SS, Foster E, Shoor S and Steimle AE. Short-term and lon- term success of electrical cardioversion in atrial fibrillation in managed care system. Internationa lArchives of Medicine 2009; 2(39):1-9 BioMed Central.
- Walsh SJ, Glover BM and Adgey J. The role of biphasic shocks for transthoracic cardioversion of atrial fibrillation. Indian Pacing and Electrophysiology Journal 2005;5(4):289-295.
- Page RL, Kerber RE, Russell JK, Trouton T, Waktare J and Gallik D. Biphasic vesus Monophasic Shock waveform for conversion of atrial fibrillation: The results of an International Randomised Double Blind Multicenter trial. Journal of the American College of Cardiology, Elsevier Science Inc.
- Ricard P, Levy S, Boccara G, Lakhal E, and Bardy G. External cardioversion of atrial fibrillation: comparison of biphasic versus monophasic waveform shocks. Europace 2001; 3:96-99.
- Mittal S, Ayati S, Stein KM et al. Transthoracic cardioversion of atrial fibrillation: compariosn of rectilinear biphasic versus damped sine wave monophasic shocks. Circulation 2000; 101:1282-1287.
- Levy S, Lauribe P, Dolla E et al. A randomized comparison of internal and external cardioversion of chronic atrial fibrillation. Circulation 1992; 86:1415-1420.
- Reisinger J, Gstrein C, Winter T, Zeindlhofer E, Hollinger K and Mori M et al. Optimization of initial energy for cardioversion of tacharryhythmias with biphasic shocks. American Journal of Emergency Medicine 2010;28:159-165 Elsevier Inc.
- Stec S, Krynski T and Kulakowski P. Efficacy of low energy rectilinear biphasic cardioversion for regular atrial tachyarrhythmias. Cardiology Journal 2011;18(1):33-38 Via Medica.
- Daubert JP and Sheu S-S. Mystery of Biphasic Defibrillation Waveform Efficacy: Is it Calcium? Journal of American College of Cardiology 2008; 52(10):836-838. Elsevier Inc.
- Hwang G-S, Tang L, Joung B et al. Superiority of biphasic over monophasic defibrillation shocks is attributable less intracellular calcium transient heterogenecity. Journal of American College of Cardiology 2008; 52(10): 828-835 Elsevier Inc.
- Niebauer M et al. Comparison of the rectilinear biphasic waveform with the damped sine monophasic waveform for external cardioversion of atrial fibrillation and flutter. American Journal of Cardiology 2004;93:1495-1499.
- Kirchhof P, Minnig G, Wasmer K, Heinecke A, Breithardt G, Eckardt L et al. A trial of self adhesive patch electrodes and hand held paddle electrodes for external cardioversion of atrial fibrillation: a multicenter comparison of antero-apical and anterp-posterior pad positions. European Heart Journal 2005; 26: 1298-1302.
- Zoll Medical Corporation. Key to successful cardioversion. 2009.
- Taylor RB. Family Medicine: Principles and practice. 6th ed. 2003 Springer.
- Boriani G, Diemberger I, Biffi M et al. Electrical cardioversion for persistent atrial fibrillation or atrial flutter in clinical practice: predictors of long-term outcome. International Journal of Clinical Practice 2007; 61(5): 748-756.
- Blich M and Edoute Y. Electrical cardioversion for persistent or chronic atrial fibrillation: Outcome and clinical factors predicting short and long term success. International Journal of Cardiology 2006;107:389-394. Elsevier Inc.
- Gallagher MM, Guo XH, Poloniecke JD, Guan Yap Y, Ward D and Camm AJ. Initial energy setting, outcome and efficiency in direct current cardioversion in atrial fibrillation and flutter. Journal of American College of Cardiology 2001; 38(5): 1498: 1504.
- Al-Ahmad A, Ellenbovices. gen KA.Natale A and Wang P.J. Pacemakers and implantable cardioverter defibrillators: An experts’ manual. Cardiotext Publishing 2010.
- Hayes DL and Friedman PA. Cardiac Pacing, defibrillation and resynchronization: A clinical approach. John Wiley and Sons. 2008.
- Ellenbogen KA and Wood MA. Cardiac Pacing and ICDs. 2005 4th ed. Wiley Blackwell.
- Burstein B and Nattel S. Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. Journal of American College of Cardiology 2008;51: 802-809.
- Elesber AA, Rozales AG, Herges RM, Shen WK, Moon BS, Malouf JF et al. Relapse and mortality following cardioversion of new onset versus recurrent atrial fibrillation and atrial flutter in the elderly. European Heart Journal Published online ahead of print, 2006.