Introduction
Breast cancer epidemiology
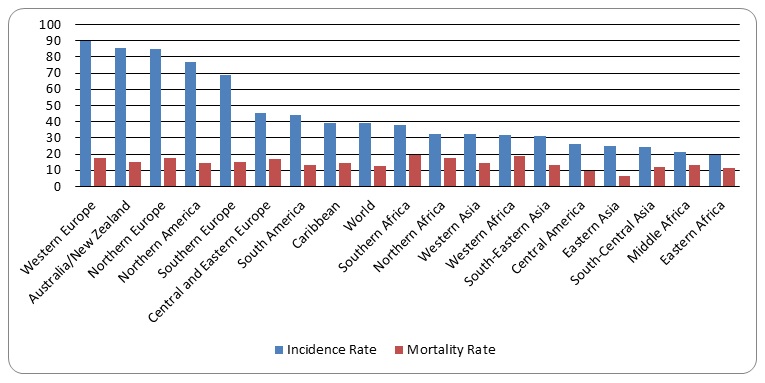
According to Breast Cancer Care, about 55,600 persons are confirmed to have breast cancer in the United Kingdom on an annual basis. It is estimated that about 500,000 mortality cases are attributed to breast cancers annually. In comparison to other parts of the world, Europe is leading in breast cancer incidents and mortality rates (Figure 1). In fact, the graphs shows that the four major regions of Europe have incidence and mortality figures that are higher than the average world figures. No research has established the reasons for the relatively high number of breast cancer cases in Europe.
Breast cancer treatment
However, typical therapies for breast cancer are invasive and, as many can attest, physically devastating. In most cases, the treatment for breast cancer involves combining resection, chemotherapy and radiotherapy (Goldhirsch, Ingle, Gelber, Coates, Thürlimann & Senn 2009). However, these treatment methods are not effective, and they leave patients with undesirable side effects that cause them to undergo much suffering. The type of treatment offered to breast cancer patients is determined by the diagnosis of the cancer cells. For example, a growth in the breast muscle detected early could be removed via minor surgical procedures. However, cases that are noted in advanced stages need to be managed through surgery, chemotherapy, and radiotherapy. However, at very advanced stages, it is eared that surgery could lead to faster spread of cancer cells, a process that could lead to more clinical and physiological symptoms that lead to death. There has been little improvement in the survival rates of most breast cancers in recent decades (King et al 2001; Kriege et al 2004).
Genes associated with breast cancer and its progression
Research shows that if the BRAC1 and BRAC2 genes mutate, then breast cancer sets in (Chen & Parmigiani 2007; King, Marks & Mandell 2003; Rebbeck, Kauff & Domchek 2009; Zhang et al 2009). These are the two genes that code for breast cancer proteins that are exhibited in the form of growths. The expression of the genes is determined by molecular events that could be target for pharmacological interventions. The confirmed origins of breast cancer are the milk ducts or lobules, before spreading and affecting the breast tissue. Breast cancer is first noticed when a lump appears on he breasts. Mammogram is used to identify the earliest forms of the breast cancer (Kriege et al 2004). Other observations are also used to indicate the onset of breast cancer in humans. The common indications for breast cancer are: abnormal nipples, thickened breast tissue and breast pain, among others. In some cases, it is difficult to diagnose breast cancer because its symptoms resemble those that are expressed in other diseases and health conditions. However, histological tests involving the study of the breast tissue is the definitive test that confirms whether or not breast cancer is affecting an individual.
Cancer cells migrate from the breast tissues to other tissues and organs where they cause damage. The rates at which breast cancer cells migrate to other body regions vary among individuals. This could be explained by genetic and other individual factors like immune responses. Therefore, persons with weak immune responses could be characterised by faster rates of cancer cell migration than individuals with stronger immune responses that recognise and kill cancer cells.
Risk factors
Breast cancer is attributed to risk factors to which an individual might be exposed. For example, aging in women has been demonstrated to correlate positively with the chances of developing breast cancer (Ganz, Greendale, Petersen, Zibecchi, Kahn & Belin 2000). However, there has been a drop in the average age at which breast cancer could affect individuals. Genetics have been demonstrated to influence the rate of human breast cancer development. Persons whose family members have had breast cancers are at increased risks of developing breast cancer in life. This happens through hereditary genetics, which perpetuates the inheritance of the breast cancer along the family lines (McPherson, Steel & Dixon 2000). Breast cancer screening of such individuals is encouraged so that, in case they are found to have abnormal growths in the breast, then appropriate measures can be taken and hinder from having serious cases of breast cancer.
Lifestyle could contribute to breast cancer (Dal Maso 2008). Smoking influences the rate at which human breast cancer develops in humans. Smoke could have chemicals that trigger molecular events that culminate in breast cancer (Secretan et al 2009). The molecular events are mediated by the BRAC1 and BRAC2 genes discussed earlier in this report. It has also been found that women who breastfeed in their lives have lower chances of suffering from breast cancer than those who do not breastfeed. Alcohol intake, high fat intake and obesity have been shown to lead to high chances of developing human breast cancer.
Prevention
Breast cancer prevention initiatives have been geared towards encouraging persons to avoid exposures to lifestyles that are known to result in breast cancer. In fact, evaluation of such strategies has shown that persons who adopt healthy lifestyles have fewer chances of developing breast cancer. The study focused on determining the major compounds extracted from devil’s club and their anticancer biological activities. The study also aimed at determining the efficacy levels of the extracts from Devil’s Club on breast cancer cells.
Justification of the study
Human breast cancer continues to be a major killer of women and men worldwide. There is no preventive vaccine for the human breast cancer, and the management of the disease depends on the stage at which it is identified in an individual (Basu et al 2006; Disis et al 2009). Mittendorf et al (2007) conducted research to understand issues that were related to research of an effective breast cancer vaccine. The researchers analysed the topic by reviewing literature publishing in previous studies. They found out that there were several approaches that could be adopted for the development of an effective breast cancer vaccine. Some of the identified approaches were based on tumour dendritic cell fusion and DNA manipulations (purified antigens or DNA portions). However, most of the identified potential vaccines did not pass preclinical testing because they did not demonstrate a significant clinical response. A lot of emphasis is directed towards designing a preventive prophylactic agent that could not pose harm to immunocompetent individuals. Clinical trials are investigating the efficacy of vaccines targeting tumour-associated antigens.
Kriege et al 2004) note that the management approaches that are based on chemotherapies are quite expensive for the ordinary person. The current treatment approaches are invasive and cause many undesirable effects to humans under therapy (Disis et al 2009; King et al 2001) For example, radiotherapy and chemotherapy cause severe cases of loss of appetite and loss of hair, among others. There has been a shift by persons suffering from breast cancer to alternative methods of treatment.
Boon et al (2007) studied the trends of options for chemotherapeutic agents by individuals suffering from breast cancer (n =557) and 2005 (n=877). The group of researchers used surveys to collect their data. In 1998, the number of respondents who responded to the survey questions was 76.3% while in 2005 the response rate was 63%. The surveys targeted cancer survivors in Canada. About 81.9% of female cancer patients were demonstrated to have used a complimentary alternative medicine or visited an alternative medicine therapist while in 1998 the percentage was 66.7% (p = 0.0002). The figures for the usage of the alternative medicine were sixty two percent and 70.6% in 1998 and 2005 respectively. In 1998 and 2005 the figures were 39.4% and 57.4 respectively. The study established that the shift to the alternative medicine was significant. In particular, 41% of women were shown to have used alternative medicine in the management of breast cancer. The products used in the management of breast were demonstrated to be green tea and vitamins C and E while the therapists were nutritionists and message therapists.
Research shows that alterations in DNA sequences in genes are responsible for breast and other types of cancers. The changes are as a result of cell molecular events that are mediated by several molecules that participate in important cellular pathways. The mutated cells have abnormal morphological features and biological properties which are indicated by pathophysiological presentations (Minn et al 2005). Some of the molecular changes that result in abnormal shapes of cells also cause cells to grow and multiply abnormally. Another feature of cancer cells is that they migrate and invade the surrounding tissues where they disrupt normal cellular events (Secretan et al 2009)
Oplopanax horridus (Devil’s club) grows mainly in North America (its image shown in figure 2). It is eaten by deer, moose, elk, and bears. The inner bark of the root is what humans most commonly use, and Devil’s bark has been used by Tlingit and other indigenous people for thousands of years in the treatment of arthritis (analgesic,) diabetes, and low blood sugar, among others. Teas made from the plant have been used to treat respiratory ailments, including tuberculosis. The plant can also be used as a laxative and emetic. The berries can be made into a paste that kills lice. Thus, the plant was used to cure a very many ailments in the areas where it grew naturally as a shrub.
The numerous uses of the extracts from the plant led to the assumption that it could have compounds that could have breast cancer cell killing properties. A recent study on Oplopanax horridus isolated a total of thirteen compounds in the plant, (various polyynes, sesquiterpenes, steroids, and phenolic acids) and then cancer killing effects of the agents under investigation. The results of the study were that the two compounds “significantly” induced cell killing and cell cycle arrest two stages of the cell cycle. Pro-apoptotic effects are biological properties of compounds that promote cell death or arrest of growth that culminates in eventual cell death. The S and G2/M phases are important in cell division and growth. If the phases are interfered with, then cells could not divide and they would reduce in number. The reduction in the number of cells happens due to continued apoptotic events that are balanced with cell division events. The study aimed at understanding the apoptotic events and their implications for breast cancer cells. The study examined the effects on human breast cancer and colorectal cells (Wang et al 2013).

Main objective
To investigate the anticancer properties of O. Horridus on human breast cancer.
Specific objectives
The study aimed at achieving the following specific objectives: First, it aimed at determining the anticancer properties of the various compounds found in O. horridus. Second, the report aimed at comparing the effects of the whole O. horridus and its isolates on human breast cancer cells. Third, it aimed at comparing the anticancer properties of O. horridus isolates from the berries and bark on human breast cancer. Fourth, it focused on determining the current treatment approaches in the control of the genetic-based disease. Lastly, it aimed at determining the scientific and economic reasons for adopting O. horridus in the control of the genetic-based disease.
Methods
The following search engines were used to do internet searches: Google, Yahoo, MSN, and Google Scholar to search for the articles in the following databases: PubMed and Hinari. Attempts were made to download and save PDF versions of the articles for easy access on the computer. The specific search terms used were: human breast cancer, treatment of cancer, Oplopanax horridus, antiproliferative, herbs on breast cancer. The terms were used in different combinations like ‘treatment of human breast cancer’, treatment of human breast cancer using O. horridus’, etc. Only studies published in English language between 2002 and 2013 were reviewed. The English language was preferred because it was easily understood by the researcher. The period of time, i.e. between 2002 and 2013, was selected so that the results could be recent and relied upon in making inferences about the population with regard to the use of compounds from devil’s club in the treatment of breast cancer. Unpublished studies could not be considered because they were not yet peer-reviewed. The searches were aimed at identifying published studies on O. horridus and human breast cancer.
Studies were only selected if they met the following criteria: (i) the research study utilized human or animal subjects, (ii) the study used the a compound derived from O. Horridus, (iv) the study investigated any other form of cancer studied in the last ten years, and (v) the study was carried out within the last 11 years (to ensure that findings were recent). Any study that did not meet all the requirements or parts of the criteria was not selected.
The following information was looked at in the studies selected: Year of publication, study location, objectives and aims of the study, study hypotheses, results, discussion and conclusion.
Results and discussion
Breast cancer dynamics
It is projected that over one million persons across the world develop breast cancer every year (McPherson, Steel & Dixon, 2000). Among all malignant and benign tumours in women, breast cancer is the most common killer. Also, about 14000 females die of breast cancer every year across the world. Research demonstrates that aging positively correlates with the chances of developing breast cancer. In fact, males and females aged 50 years and above have more chances of being diagnosed with breast cancer than those aged below 50 years. MacMahon (2006) associated breast cancer epidemiology with the following 3 distinct characteristics: chances of developing the disease increase with childbearing, its development closely related to the activities of the ovary, and international differences in the ages of persons developing the disease. The researchers studied breast cancer development dynamics to understand the three features of interest. The study authors conducted the study in many countries across the world.
The authors note that childbearing correlates positively with the chances of developing breast cancer. This could be linked with the activities of the ovary. Thus, hormonal activities have implications for the development of breast cancer. The authors also assert that there are no significant differences in international ages for individuals developing breast cancer. Significant age variations could be attributed to differences in ethnicities and environmental factors. Kemeny et al (2003) has shown that, in clinical trials, several barriers hinder successful chemotherapy breast cancer in older female patients. Older women are more affected by the disease. Thus, treatment approaches have always been aimed at circumventing the barriers to treatment in the older women. In order to implement successful breast cancer treatments, pharmacological agents should focus on molecular pathways that lead to the establishment of the disease. However, such treatment approaches should take into consideration the side effects that could impact the old women negatively. This is because old people have higher chances of exhibiting drug side effects than the younger people due to reduced immune responses and higher chances of developing drug.
Breast cancer genetics
The mutations that occur in the two genes (BRAC1 and BRAC2) have been cited to be the genesis of both breast and ovarian cancers (Peto et al 1999). The researchers established that BRAC 1 and BRAC2 have equal roles in contributing towards the development of breast cancer. However, it was shown that the two genes correlated weakly with the chances of family members developing the disease. This could imply that members who express high levels of the two genes could have different chances of developing the health condition (Walsh & King 2007). Other studies have strongly associated breast cancer with BRAC1 and BRAC2 (Nathanson, Wooster & Weber 2001; Ford & Easton1995). It has been show that breast cancer cells spread to other body tissues and organs through complex molecular pathways that involve gene expression and silencing (Sato et al 1990; Sgroi et al 1999; Schmidt-Kittler et al 2003; Minn et al 2005). The molecules that mediate metastasis are expressed at various levels among different individuals. If, for example, an individual has molecular events whose components are expressed at high levels, then the individual could be characterised by a fast rate of breast cancer development and progression.
King and colleagues (2001) investigated the implication of tamoxifen and mutated BRAC1 and BRAC2 genes in breast cancer. It was shown that the molecule, tamoxifen, significantly reduced the chances of developing breast cancer in females with mutated BRAC2 gene. On the other hand, the molecule did not exhibit any significant roles in reducing the chances of developing breast cancer in females with mutated BRAC1 gene. Introduction of tamoxifen in individuals with mutated BRAC1 gene resulted in increased levels of breast cancer progression. Thus, the molecule could not be used to treat breast cancer. The study was important because it pointed at the important role played by tamoxifen in arresting the growth of cancer cells characterised by mutated BRAC1 gene in the development of breast cancer. The study findings are those generated by another study in 2005 (Howell et al 200).
Alternative treatment approaches
Huang et al (2011) isolated three glycosides from root barks of Devil’s Club. The structures of the three compounds were determined using NMR and mass spectrometry techniques. The glycosides were named oplopanpheside A, B and C. The study established that the glycosides did not possess any anticancer activity against the human cancer cell lines under study. Therefore, they would not be targeted in the future in the development of cancer drugs from devil’s club activities.
Boon et al (2000) conducted a study to assess prevalence of breast cancer patients who were using alternative medicine to manage the disease. The researchers sent questionnaires women suffering from breast cancer in Ontario, Canada, in 1994 and 1995. In total, 66.7% of the respondents said that they were using alternative medicine. The major reason given for using alternative medicine by the respondents was that they were boasting their immune systems. Green tea and special foods were mostly used by the women as alternative medicine products. The study showed that the use of alternative medicine was prevalent in Canada. The researchers argued that the form of medicine could reduce symptoms associated with the disease. It was shown that the use of alternative medications was on the increase, and the medications resulted in some significant biological activities against cancer cells, which were supported by prolonged healthy lives of the cancer survivors.
Lee et al (2000) investigated the use of alternative medicine in the management of breast cancer by survivors from four ethnic origins. The ethnic groups studied were Latino, Chinese, White and Black. Study participants were women who were diagnosed with breast cancer in 1990, 1991 and 1992. Women were sampled from San Francisco, California (n-379). Data were collected through telephone interviews. Study findings showed that blacks (36%) used alternative medicine to manage breast cancer because they believe the components could give them spiritual healing. Chinese women used alternative therapy as herbal remedies (22%). It was shown that women whose origin was Latin used alternative therapy as dietary therapies (30%) and supernatural healing (26%). White women used alternative therapies as dietary requirements (35%) and physical exercises (21%). The study concluded that a significant number of breast cancer survivors in San Francisco were using alternative medicine. The results imply that a good number of patients are turning to the use of alternative medicine to manage breast cancer. This could be explained by significant efficacy rates that have been demonstrated with the use of alternative medications, and their relatively lower risks of adverse side effects.
Extracts from devil’s club
Wang et al (2013) aimed at isolating and identifying anticancer compounds in O. horridus. In their previous study, the group of researchers had identified at total of 13 compounds in the medicinal herb, 5 of which had not been characterized. Flow cytometry was used to examine the anticancer effects of the compounds along the cell cycle of the cancer cell lines. The molecular tool could be used to align cells based on their lines and assess anticancer properties of various extracts from devil’s club. Wang et al (2010) also carried out an in vivo study of the compounds using a xenograft tumour model. Their findings showed that falcarindiol and oplopantriol A had the strongest anticancer properties amongst all the compounds analysed. Particularly, falcarindiol demonstrated induction of apoptosis and disruption of cell cycle phases. The compound was also assayed on HCT-116 tumour growth of the mouse. The in vivo results were also very suggestive of strong anticancer properties of the compound. It was also demonstrated that polyynes derivatives had hydroxyl groups and double bonds that conferred them their pharmacological properties.
Efficacy of devil’s club extracts in combination with common drugs
Tai et al (2013) conducted a study to investigate the anticancer properties of O. horridus extract alone and in combination with tested drugs gemcitabine (GEM0, cisplatin (CDDP), and paclitaxel (PTX) against human pancreatic cancer. Rotary cell culture system was used to prepare both 2D monolayer and 3D spheroids. It was demonstrated that the 3D spheroids were less killed by the Devil’s Club extract, GEM and PTX than the 2D monolayer of cells. The IC50 values of the concentrations of CDDP and GEM were decreased when the therapeutic agents were combined with the Devil’s Club extract. The extract was identified as a polyacetylene and it was shown to significantly enhance the anticancer properties of chemotherapeutic drugs against pancreatic cancer cells. The findings demonstrated that the assayed extract in combination with chemotherapeutic agents could be tested in vivo for assessment of anticancer properties of compounds.
Hydrophobic components
Sun et al (2010) investigated the anticancer properties of hydrophobic extracts from the bark of Devil’s Club. Chromatographic techniques were utilised to extract the chemical components from the plant bark. Compound identification was done using comparison methods, which correlated data obtained with data available in online databanks.. The following compounds were isolated:
- 9, 17-octadecadiene, 14-dyne-1, 11, 16-triol, 1-acetate
- Oplopandiol acetate
- Falcarindiol
- Oplopandiol
- Trans-nerollidol
- t-cadinol
Anticancer properties of the isolated compounds were assessed against breast cancer cell lines (MCF-7), HCT-116 cells and colon cancer cells (SW-480). All the isolates killed breast and colon cancer cells in the laboratory. However, falcarindiol demonstrated the strongest activity on the two forms of cancer cells. Flow cytometry analysis was used to show that the first four compounds in the above list induced HCT-116 cell division in the G2/M phase. Polyacetylenes exhibited apoptotic properties by inhibiting cell cycle at specific stages.
Stem versus bark extracts
Tai et al (2006) investigated the anticancer properties of root bark extracts from Devil’s Club on ovarian cancer in vitro. The Devil’s Club extracts were profiled using high performance liquid chromatography (HPLC). The group of researchers used the crystal violet assay to test for anticancer properties of the extracts from Devil’s Club. The extracts were tested alone or in combination with the following chemotherapeutic agents: cisplatin and paclitaxel. Four ovarian cancer cell lines were developed and used for the crystal violet assay. The study findings showed that Devil’s Club extracts had anticancer properties on cell lines that were Cisplatin sensitive and resistant cells were all killed chemical compounds isolated from O. horridus. Flow cytometry and microscopy analyses demonstrated that cell cycle events were blocked in the S and G2/M phases. An increase in the concentration of the extracts positively correlated with the degree of apoptosis.
Wang et al (2010) used HPLC to investigate the difference in anticancer properties between stem and berry extracts from Devil’s Club. Different human cancer lines were used in the study using the MTS assay method, i.e. breast cancer lines, ovarian cancer cell lines and lung cancer cell lines. Flow cytometry analysis was the platform upon which apoptotic and cyclin A activities were assessed. The study confirmed that extracts from the stem have more chemical compounds than those found in the berries. In addition, the stem extract showed stronger anticancer properties than the berry extract. The stem extract showed significant induction of cyclin A expression and arresting of cancer cell proliferation in S and G2/M phases. The group of researchers concluded that the properties of the stem extract in arresting cell cycle and inducing apoptosis could be essential in cancer chemoprevention. The importance of cyclin A in regulation of cell cycle has been cited. Particulary, cyclin A has been shown to regulate cell cycle for the S-phase to go beyond the G2/M phase. It has been demonstrated that cyclin A positive cells increases in cells that are treated with stem extracts of devil’s club (Figure 5). The implication is that the genes responsible for producing cyclin A are activated by the drug pressure to be increase the rate of expression. An increase in the rate at which cyclin A genes are expressed culminates in an increase in the number of cells have cyclin A molecules.
In 2012, You et al conducted high performance liquid chromatography- mass spectrometry (HPLC-MS) analyses and investigation of anticancer properties of compounds from Devil’s club. Compounds were isolated using liquid-liquid re-extraction after which their anticancer properties were assayed in vitro using cell proliferative assays. The antioxidant activities of the extracts were assessed by determining their phenolic content, ORAC value and the rate at which the compounds scavenged cells (DPHH activity). All the cancer cells lines that were inhibited were shown to be arrested and killed by the phenolic compounds. Therefore, it could be suggested that Devil’s club extract’s antiproliferative properties were as a result of the action of phenol containing compounds.
Tai et al (2006) assessed the anticancer and antioxidant properties of compounds extracted from Devil’s club. The group of researchers used HPLC profiling to extract different compounds of Devil’s club. In particular, they used dry root bark powder for the in vitro analysis. The IC50 values of the extract were estimated to know the minimum concentrations of the extract that could inhibit cell growth and proliferation. Concentrations of the extract below the established IC50 were combined with concentrations of PTX and camptothecin below IC50. The combination of the concentrations increased the antiproliferative properties of the drug concentration on K562 cells. In summary, 9 combinations demonstrated additive effects while 10 combinations showed antagonistic effect. The Devil’s club extract showed significant antioxidant activity by scavenging cells in a cell free scavenging assay. The overall results obtained in the study showed that the ethanolic extract of Devil’s club had anticancer properties on many cancer cell lines and significant antioxidant properties.
Kobaisy et al (1997) conducted a study to determine the identities of polyynes found in Devil’s Club that could be used to kill mycobacteria. All the five polyynes isolated were found to kill Candida, bacteria and mycobacteria in vitro. In addition, the polyynes were shown to have the in vitro efficacy against Mycobacterium tuberculosis. The following polyynes were identified:
- Falcarinol,
- Oplopandiol,
- 9,17-octadecadiene-12,14-diyne-1,11,16-triol 1-acetate,
- Falcarindiol, and
- Oplopandiol acetate.
Oplopandiol acetate and oplopandiol were the novel polyynes while the other three polyynes were characterized in previous studies. The researchers concluded there could be other compounds of medicinal value. The impact of the findings to the current research study is that several chemical compounds could be extracted from devil’s club based on the study aims. They could poses little or significant effects on breast cancer cells.
McCutcheon (1995) conducted a wide study with the aim of determining anticancer activities of plants. About one hundred methanol-containing plant extracts were assayed in vitro to determine the killing efficacies against seven different types of viruses. Only twelve compounds were shown to kill the viruses at the non-cytotoxic concentrations. Extract isolated from the inner bark of Devil’s Club was found to have partial antiviral activities.
Hassan et al (2012) conducted a review of plants found to have medicinal value in Northern Ontario. It was shown that extracts isolated from the inner bark of Devil’s Club had shown significant activities against cancer cell growth and proliferation, bacteria, viruses, and mycobacteria. The review contributed to the knowledge of Devil’s Club medicinal value against a range of diseases across the world.
Excited by the growing evidence of medicinal value of extracts from the inner bark of the root and stem from Devil’s Club, Gruber et al (2004) developed HPLC and TLC assays that could be used for isolation of the Devil’s Club extracts. The development and validation of the two assays was essential because they became the two most widely accepted methods of isolating medicinal extracts from Devil’s Club. With the two assays, scientists could isolate, characterize and identify isolate compounds from Devil’s Club.
Huang et al (20010) conducted a study in which they isolated and identified two polyynes from Devil’s Club. The new compounds were named oplopantriol A and oplopantriol B. The compounds were given the names based on their chemical structures. The two novel compounds were isolated from the root bark of Devil’s Club. Mass spectroscopic and NMR analyses were utilised to elucidate the identities of compounds. The data from these techniques were compared with those of known structures to elucidate the real structures of the isolated compounds. The anticancer properties of the two compounds have not been assessed. However, they could have anticancer properties because their structurally related compounds from the root bark of Devil’s Club have been shown to kill cancer cells in vitro.
Jin et al (2012) were motivated by the increasing evidence of the anticancer properties of falcarindiol. The group of researchers aimed at deciphering the mechanisms through which the compound promotes cell killing of the cancer cells. They relied on the findings from a previous study which had shown that the compound could kill only colon cancer cells, leaving behind the healthy colon cells intact. Jin et al (2012) were able to establish that the compound killed cancer cells by inducing endoplasmic reticulum (ER) stress and initiating unfolded protein response. When the ER stress was reduced in cells, there was minimal cell death. However, when the ER stress was increased in the cancer cells under investigation, the rate at which the cells were killed was increased. It was concluded that the anticancer agent demonstrates its anticancer activities by promoting ER stress and apoptosis.
Li et al (2010) investigated the in vitro efficacy of extracts from Devil’s Club on human colorectal cancer cells. The research findings established that Devil’s Club extract and its fractions shown anticancer properties against human colorectal cells. The researchers concluded that the compounds acted by apoptosis and cell cycle alterations. The duo actions could arrest cancer cell growth and proliferation. The phases of the cell cycle that were affected were the M and G2/M phases. The arrest of the cell cycle at these phases altered the normal cell cycle processes, which resulted in cell death, i.e. induced apoptosis. The findings were similar to those obtained in another study regarding the activity of extracts from devil’s club on abnormal colorectal cells (Tai, Cheung, Chan & Hasman 2010)
Sun et al (2010) aimed at promoting antiproliferative properties of extracts from the root bark of Devil’s Club by eliminating lipophilic components. Lipophilic components are compounds that are soluble in water. The study utilised cells isolated from breast and lung tissues. In particular, the researchers examined the effects of Devil’s Club extract on the cell cycle and apoptosis of cancer cells. The study authors aimed at deciphering the impact of introducing various doses of devil’s club extracts on breast and lung cancer cells. The research findings demonstrated that using water free extracts could enhance anticancer activities of extract from Devil’s Club. The study assays used ethanol based drug solutions to replace water-soluble components. Increasing the amount of ethanol containing components correlated positively with antiproliferative properties of the Devil’s Club’s extract. The findings have important implications for the development anticancer drugs from devil’s club in the future. For example, it would be essential to have the minimum amount of lipophilic compounds in the treatment combinations involving the use of devil’s club extract. However, the development of such drugs would focus on achieving safe concentrations of ethanol for effective biological effects because too much ethanol could result in poisoning of healthy tissues and cells.
Inui et al (2007) used the counter-current chromatography to isolate extracts from Devil’s Club that were used to test for their synergetic effects against mycobacterium tuberculosis in vitro. The researchers assumed that mixing the components derived from the plant could go a long way in enhancing their efficacy, increasing bioavailability of the components and minimising side effects. It was established that some combinations of the extracts from the plant could result in synergetic effects against mycobacteria. However, some other combinations resulted in reduced levels of mycobacteria killing biological activities. The research study provided a basis upon which combinations of compounds isolated from Devil’s Club could be used to produce synergistic effects in vitro, and possibly in vivo. For example, it would be important to identify the combination of compounds with the highest levels of synergetic activities because it would lead to the achievement of the best anticancer killing effects.
Oil components and glycosides
Garneau et al (2006) investigated the oil components found in Devil’s Club. Extracts derived from the stem and root barks of the medicinal plant were evaluated for oil composition. The researchers used gas chromatography and mass spectrometry for the analysis. In both the extracts from the stem and root bark, (E)-nerolidol was the major component found. However, the researchers did not aim at the efficacy of the oil extract breast cancer cells. In the future, it would be important to assess the activity of such oil extracts on cancer cell killing.
Efficacy of chemical extracts from devil’s club
It is probably important to compare the efficacy of the chemical compounds extracted from the berry and bark root of devil’s club so as to determine the portion of the plant that has chemicals with better anticancer properties (Figure 3).
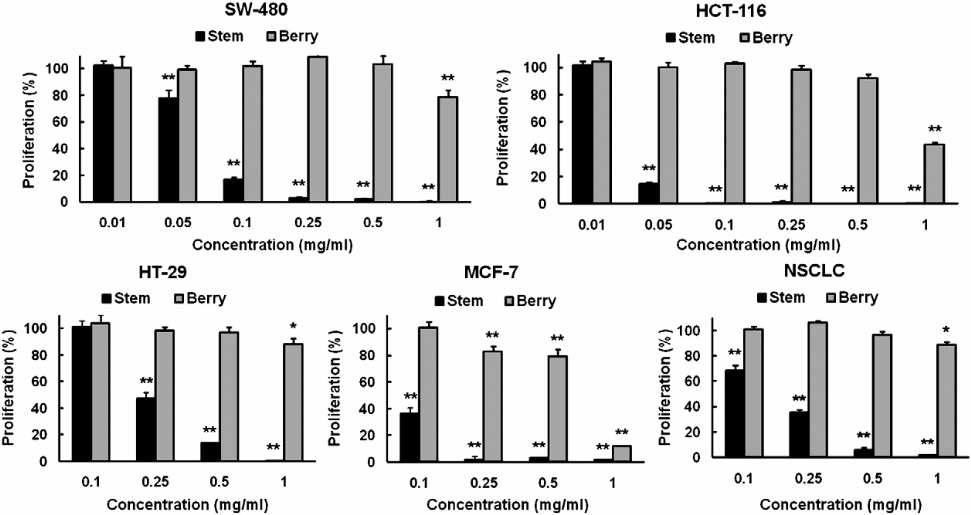
The figure was chosen because it contains comparisons of the efficacies of the different concentrations of the extracts obtained from both the bark and stem parts of devil’s club. The cell lines assayed were NSCLC, HT-29, MCF-7, SW-480, and HCT-116. From the above graphical representation, it is clear that the extracts from the stem have more anticancer potency than those from the berry. In fact, they have similar anticancer properties with extracts from the root bark of devil’s club. It was important to establish the source of devil’s club compounds that have significant anticancer biological activities so that research in the future would focus on the particular source(s).
To further show the implication treating SW-480 cells with stem extracts, it would be important to compare the cell analysis results obtained though cell flow cytometry (Figure 4).
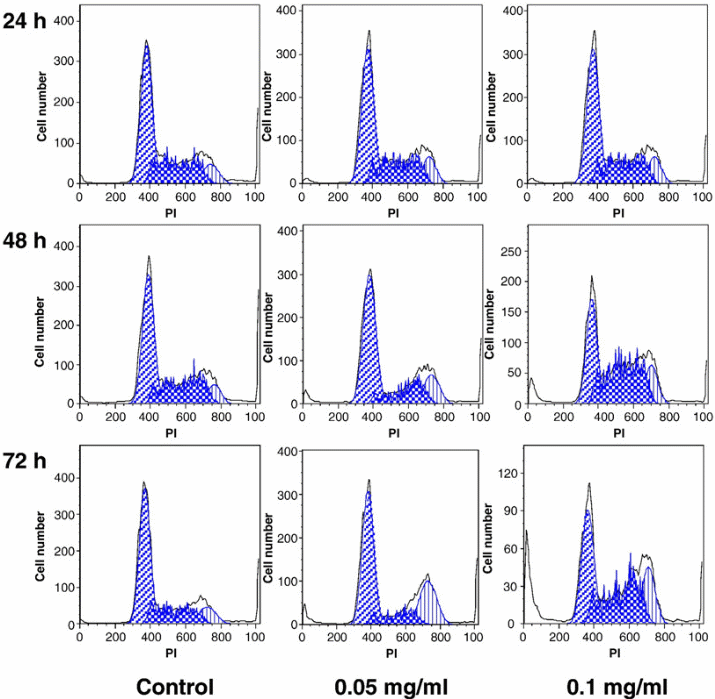
The figure demonstrates the implication of a higher concentration of the stem extract on the viability of SW-480 cells. The assumption here is that the activity of the stem extracts on the SW-480 cells would be similar to that on the breast cancer cells (MCF-7). The control was not expected to change because it was used to act as a standard. However, the figure demonstrates that if the concentration of the chemical extract increases, then the number of the cancer cells killed per unit time also increases. This would be essential in the future when designing anticancer drugs from the medicinal plant. The design would focus on the production of drugs at specific strengths aimed at killing cancer cells at different stages. An average amount of drug with sufficient killing strength, but with minimal side effects would be the ideal dose.
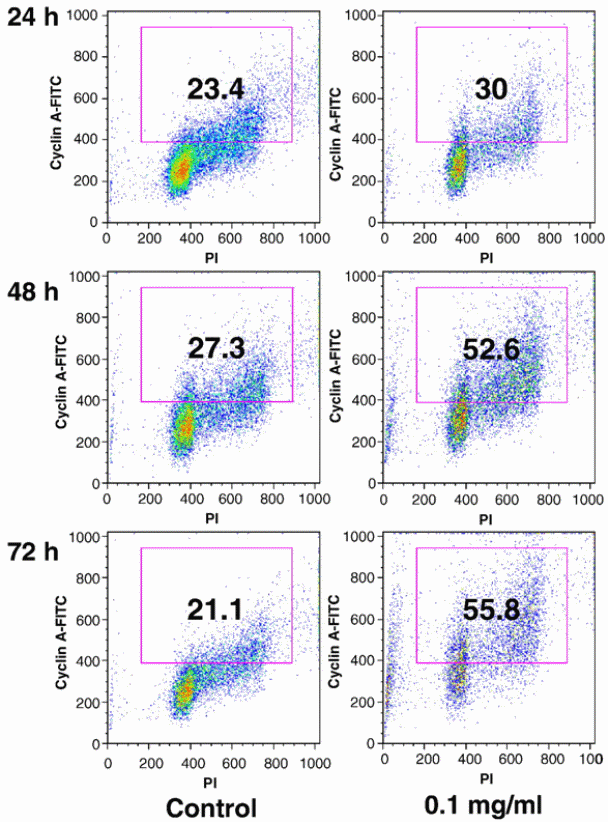
The importance of cyclin A in regulation of cell cycle has been cited. Particulary, cyclin A has been shown to regulate cell cycle for the S-phase to go beyond the G2/M phase. It has been demonstrated that cyclin A positive cells increases in cells that are treated with stem extracts of devil’s club (Figure 5). The implication is that the genes responsible for producing cyclin A are activated by the drug pressure to be increase the rate of expression. An increase in the rate at which cyclin A genes are expressed culminates in an increase in the number of cells have cyclin A molecules.
The major compounds from devil’s club
Research has established that devil’s club has 13 major compounds that could be extracted using various chemical methods. However, only 6 compounds among the 13 compounds have been shown to have anticancer activities.
Figure 6: Major groups of the 13 compounds isolated from devil’s club.
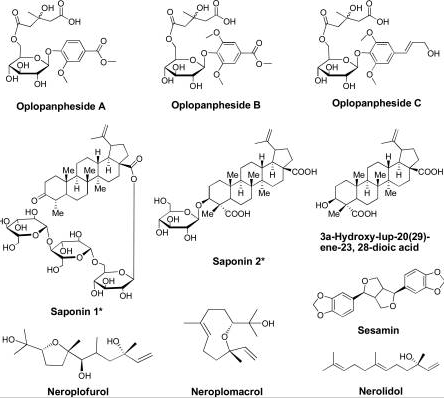
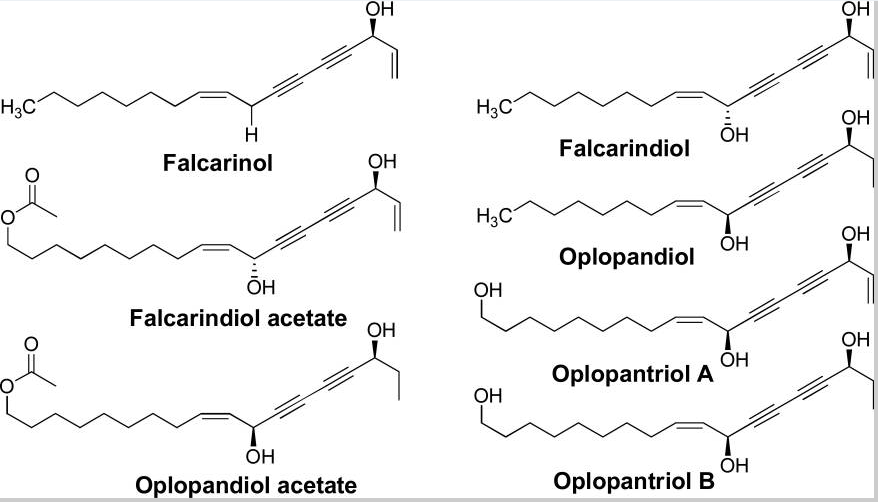
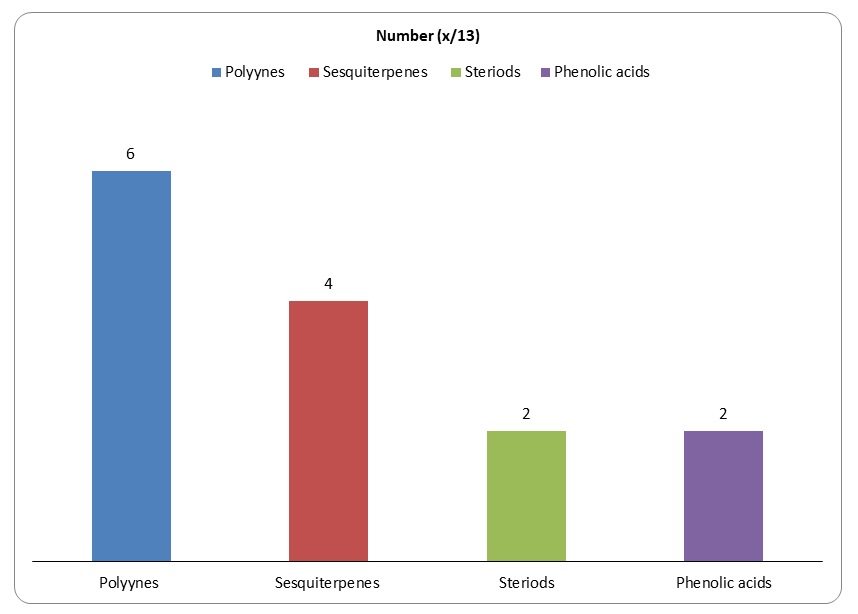
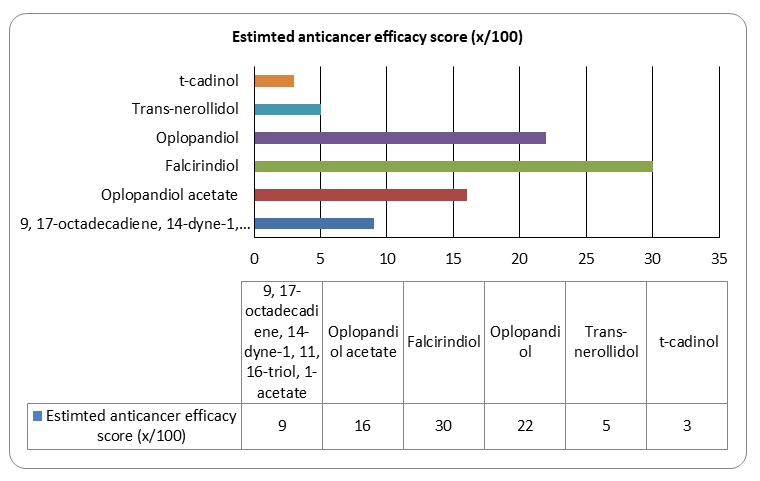
It would be important to summarise the anticancer efficacy levels of the major polyynes identified in devil’s club. As Figure 6 shows, falcarindiol has the greatest anticancer activities while has the least anticancer properties. Oplopandiol has been shown to have the second best anticancer properties. The mode of action of falcarindiol and oplopandiol has been shown to be almost similar. Specifically, the two compounds exhibit their cancer-killing activities by interfering with cell division events, a process that culminates in the arrest of cell growth and eventual death. This is the main target of drugs that are designed to kill cancer cells of various origins.
Conclusion
Breast cancer is a major public health problem across the world. The health condition does not have a cure or an active vaccine. It affects both males and females. Breast cancer has been attributed to the expression of BRAC1 and BRAC2 genes which initiate the initiation of the condition. The two genes code for the breast cancer proteins 1 and 2. However, it has been established that the two genes are expressed differentially in different persons. Molecular pathways involved in the initiation and progression of breast cancer are mediated by molecules that are expressed at different rates in many individuals. The current treatment methods for breast cancer are often invisible and involve many side effects, some of which could have devastating effects on individuals. Initiation of breast cancer has been shown to correlate with genetics.
Devil’s club is a shrub growing in North America and it has been used over the years to treat many diseases, for example, cold flu and diabetes. Recently, the traditional herb has been studied in vitro to determine the cancer killing activities of its extracts. The article analyses conducted in this report establish that flow cytometry is a perfect molecular too for studying the biological effects of devil’s club extracts while HPLC is used to extract various compounds from the plant. Refer to section 3.4 for the thirteen (13) compounds have been extracted from devil’s club.
As mentioned in Figure 10 in section 3.4 Falcarindiol and oplopandiol show the highest levels of cancer killing activities. The killing activities of the compounds occur through interference of phases of cell division, a process that culminates in cell apoptosis. In vitro studies have shown sufficient evidence that devil’s club compounds have significant biological effects on breast cancer cells. This report shows that the extracts from the shrub could be used commercially to cure breast cancer. This will involve many in vivo studies characterised by the four phases of clinical trials. If the clinical trials will provide sufficient evidence of breast cancer cell killing activities using devil’s club, then the compounds from the plant will be used in the future for the treatment of breast cancer.
References
Basu, GD, Tinder, TL, Bradley, JM, Tu, T, Hattrup, CL, Pockaj, BA, & Mukherjee, 2006, “Cyclooxygenase-2 inhibitor enhances the efficacy of a breast cancer vaccine: role of IDO”, The Journal of Immunology, vol. 177, no. 4, pp. 2391-2402.
Boon, H, Stewart, M, Kennard, MA, Gray, R. Sawka, C, Brown, JB.,… & Haines-Kamka, T, 2000, “Use of complementary/alternative medicine by breast cancer survivors in Ontario: prevalence and perceptions”, Journal of Clinical Oncology, vol. 18, no. 13, pp. 2515-2521.
Boon, HS, Olatunde, F, & Zick, SM, 2007, “Trends in complementary/alternative medicine use by breast cancer survivors: comparing survey data from 1998 and 2005”, BMC women’s health, vol. 7, no. 1, pp. 4.
Chen, S, & Parmigiani, G, 2007, “Meta-analysis of BRCA1 and BRCA2 penetrance”, Journal of Clinical Oncology, vol. 25, no. 11, pp. 1329-1333.
Dal Maso, L, Bosetti, C, La Vecchia, C, & Franceschi, S, 2009, ‘Risk factors for thyroid cancer: an epidemiological review focused on nutritional factors’, Cancer causes & control, vol. 20, no. 1, pp. 75-86.
Disis, ML, Wallace, DR., Gooley, TA, Dang, Y, Slota, M, Lu, H,… & Salazar, LG, 2009, “Concurrent trastuzumab and HER2/neu-specific vaccination in patients with metastatic breast cancer”, Journal of Clinical Oncology, vol. 27, no. 28, pp. 4685-4692.
Ford, D, & Easton, DF, 1995, “The genetics of breast and ovarian cancer”, British journal of cancer, vol. 72, no. 4, pp. 805.
Ganz, PA, Greendale, GA, Petersen, L, Zibecchi, L, Kahn, B, & Belin, TR, 2000, “Managing menopausal symptoms in breast cancer survivors: results of a randomized controlled trial”, Journal of the National Cancer Institute, vol. 92, no. 13, pp. 1054-1064.
Garneau, FX, Collin, G, Gagnon, H, Jean, FI, Strobl, H, & Pichette, A, 2006, ‘The essential oil composition of devil’s club, Oplopanax horridus JE Smith Miq’, Flavour and fragrance journal, vol. 21, no. 5, pp. 792-794.
Goldhirsch, A, Ingle, JN, Gelber, RD, Coates, AS, Thürlimann, B, & Senn, HJ, 2009, “Thresholds for therapies: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2009”, Annals of oncology, vol. 20, no. 8, pp. 1319-1329.
Gruber, JW, Kittipongpatana, N, Bloxton, JD, Der Marderosian, A, Schaefer, FT, & Gibbs, R, 2004, “High-performance liquid chromatography and thin-layer chromatography assays for Devil’s Club (Oplopanax horridus)”, Journal of chromatographic science, vol. 42, no. 4, pp. 196-199.
Hassan, H M, Jiang, ZH, Syed, TA, & Qin, W, 2012, “Review: Northern Ontario medicinal plants”, Canadian Journal of Plant Science, vol. 92, no. 5, pp. 815-828.
Howell, A, Cuzick, J, Baum, M, Buzdar, A, Dowsett, M, Forbes, JF.,… & Tobias, JS, 2005, “Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer”, Lancet, vol. 365, no. 9453, pp. 60-62.
Huang, WH, Zhang, QW, Meng, LZ, Yuan, CS, Wang, CZ, & Li, SP, 2011, “Oplopanphesides A—C, Three New Phenolic Glycosides from the Root Barks of Oplopanax horridus”, Chemical and Pharmaceutical Bulletin, vol. 59, no. 5, pp. 676-679.
Huang, WH, Zhang, QW, Wang, CZ, Yuan, CS, & Li, SP, 2010, “Isolation and Identification of Two New Polyynes from a North American Ethnic Medicinal Plant–Oplopanax horridus (Smith) Miq”, Molecules, vol. 15, no. 2, pp. 1089-1096.
Inui, T, Wang, Y, Deng, S, Smith, DC, Franzblau, SG, & Pauli, GF, 2007, ‘Counter-current chromatography based analysis of synergy in an anti-tuberculosis ethnobotanical’, Journal of chromatography A, vol. 1151, no. 1, pp. 211-215.
Jin, HR, Zhao, J, Zhang, Z, Liao, Y, Wang, CZ, Huang, WH,… & Du, W, 2012, “The antitumor natural compound falcarindiol promotes cancer cell death by inducing endoplasmic reticulum stress”, Cell death & disease, vol. 3, no. 8, pp. 376.
Kemeny, MM, Peterson, BL, Kornblith, AB, Muss, HB, Wheeler, J, Levine, E,… & Cohen, HJ, 2003, “Barriers to clinical trial participation by older women with breast cancer” Journal of Clinical Oncology, vol. 21, no. 12, pp. 2268-2275.
King, MC, Marks, JH, & Mandell, JB, 2003, “Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2”, Science, vol. 302, no. 5645, pp. 643-646.
King, MC, Wieand, S, Hale, K, Lee, M, Walsh, T, Owens, K,… & Fisher, B, 2001, “Tamoxifen and breast cancer incidence among women with inherited mutations in BRCA1 and BRCA2”, JAMA: the journal of the American Medical Association, vol. 286, no. 18, pp. 2251-2256.
Kobaisy, M, Abramowski, Z, Lermer, L, Saxena, G, Hancock, REW, Towers, GHN.,… & Stokes, RW, 1997, “Antimycobacterial polyynes of Devil’s Club (Oplopanax horridus), a North American native medicinal plant”, Journal of natural products, vol. 60, no. 11, pp. 1210-1213.
Kriege, M, Brekelmans, CT, Boetes, C, Besnard, PE, Zonderland, HM, Obdeijn, IM,… & Klijn, JG, 2004 “Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition”, New England Journal of Medicine, vol. 351, no. 5, pp. 427-437.
Lee, MM, Lin, SS, Wrensch, MR, Adler, SR, & Eisenberg, D, 2000, “Alternative therapies used by women with breast cancer in four ethnic populations”, Journal of the National Cancer Institute, vol. 92, no. 1, pp. 42-47.
Li, XL, Sun, S, Du, GJ, Qi, LW, Williams, S, Wang, CZ, & Yuan, CS, 2010, “Effects of Oplopanax horridus on human colorectal cancer cells”, Anticancer research, vol. 30, no. 2, pp. 295-302.
MacMahon, B, 2006, “Epidemiology and the causes of breast cancer”, International journal of cancer, vol. 118, no. 10, pp. 2373-2378.
McCutcheon, AR, Roberts, TE, Gibbons, E, Ellis, S. M, Babiuk, L A, Hancock, REW, & Towers, GHN, 1995, “Antiviral screening of British Columbian medicinal plants”. Journal of Ethnopharmacology, vol. 49, no. 2, pp. 101-110.
McPherson, K, Steel, C, & Dixon, JM, 2000, “ABC of breast diseases: breast cancer—epidemiology, risk factors, and genetics”, BMJ: British Medical Journal, vol. 321, no. 7261, pp. 624.
Minn, AJ, Gupta, GP, Siegel, PM, Bos, PD, Shu, W., Giri, DD… & Massagué, J, 2005, “Genes that mediate breast cancer metastasis to lung”, Nature, vol. 436, no. 7050, pp. 518-524.
Mittendorf, EA, Peoples, GE, & Singletary, SE, 2007, “Breast cancer vaccines. Cancer, vol. 110, no. 8, pp. 1677-1686.
Nathanson, KN, Wooster, R, & Weber, BL, 2001, “Breast cancer genetics: what we know and what we need”, Nature medicine, Vol. 7, No. 5, pp. 552-556.
Peto, J., Collins, N, Barfoot, R, Seal, S, Warren, W, Rahman, N,… & Stratton, MR, 1999, “Prevalence of BRCA1 and BRCA2 gene mutations in patients with early-onset breast cancer”, Journal of the National Cancer Institute, vol. 91, no. 11, pp. 943-949.
Rebbeck, TR, Kauff, ND, & Domchek, SM, 2009 “Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers” Journal of the National Cancer Institute, vol. 101, no. 2, pp. 80-87.
Sato, T, Tanigami, A, Yamakawa, K, Akiyama, F, Kasumi, F, Sakamoto, G, & Nakamura, Y, 1990, Allelotype of breast cancer: cumulative allele losses promote tumor progression in primary breast cancer”, Cancer research, vol. 50, no. 22, pp. 7184-7189.
Schmidt-Kittler, O, Ragg, T, Daskalakis, A, Granzow, M, Ahr, A, Blankenstein, TJ,… & Klein, CA, 2003, “From latent disseminated cells to overt metastasis: genetic analysis of systemic breast cancer progression”, Proceedings of the National Academy of Sciences, vol. 100, no. 13, pp. 7737-7742.
Secretan, B, Straif, K, Baan, R, Grosse, Y, El Ghissassi, F, Bouvard, V,… & Cogliano, V, 2009, “A review of human carcinogens—Part E: tobacco, areca nut, alcohol, coal smoke, and salted fish”, The lancet oncology, vol. 10, no. 11, pp. 1033-1034.
Sgroi, DC, Teng, S, Robinson, G, LeVangie, R, Hudson, JR, & Elkahloun, AG, 1999, “In vivo gene expression profile analysis of human breast cancer progression”, Cancer research, vol. 59, no. 22, pp. 5656-5661.
Sun, S, Du, GJ, Qi, LW, Williams, S, Wang, CZ., & Yuan, CS, 2010, “Hydrophobic constituents and their potential anticancer activities from Devil’s Club Oplopanax horridus Miq”, Journal of ethnopharmacology, vol. 132, no. 1, pp. 280-285.
Tai, J, Cheung, S, Chan, E, & Hasman, D, 2010, “Inhibition of human ovarian cancer cell lines by Devil’s club (Oplopanax horridus)” Journal of ethnopharmacology, vol. 127, no. 2, pp. 478-485.
Tai, J, Cheung, S, Cheah, S, Chan, E, & Hasman, D, 2006, “In vitro anti-proliferative and antioxidant studies on Devil’s Club (Oplopanax horridus)”, Journal of ethnopharmacology, vol. 108, no. 2, pp. 228-235.
Tai, J, Cheung, SSC, Ou, D, Warnock, GL, & Hasman, D, 2013, “Antiproliferation activity of Devil’s club (Oplopanax horridus) and anticancer agents on human pancreatic cancer multicellular spheroids”, Phytomedicine, vol. 1, no. 2, pp. 1-9.
Walsh, T, & King, MC, 2007, “Ten genes for inherited breast cancer”, Cancer cell, vol. 11, no. 2, pp. 103-105.
Wang, CZ, Aung, HH, Mehendale, SR, Shoyama, Y, & Yuan, CS, 2010, “High performance liquid chromatographic analysis and anticancer potential of Oplopanax horridus: Comparison of stem and berry extracts”, Fitoterapia, vol. 81, no. 2, pp. 132-139.
Wang, CZ, Zhang, Z, Huang, WH, Du, GJ, Wen, XD, Calway, T,… & Yuan, CS, 2013, “Identification of potential anticancer compounds from Oplopanax horridus”, Phytomedicine, vol. 20, no. 1, pp. 999-1006.
You, Q. Chen, F. Ni, H, Wang, X, Jiang, Y, & McCoy, JAH, 2012, “HPLC–MS analyses and bioactivities of novel chemicals in Devil’s club (Oplopanax horridus) Sm. Miq”, Food Chemistry, vol. 135, no. 1, pp. 199-207.
Zhang, F, Ma, J, Wu, J, Ye, L, Cai, H, Xia, B, & Yu, X, 2009, “PALB2 links BRCA1 and BRCA2 in the DNA-damage response” Current Biology, vol. 19, no. 6, pp. 524-529.